New HBV-targeted TCR T Cell Therapy (SCG101) Shows Improved Survival in Liver Cancer: EASL Conclusion
SCG Cell Therapy Pte Ltd, a biotechnology firm focused on clinical-stage development of innovative immunotherapies for infectious diseases and related cancers, revealed that groundbreaking clinical data from its unique autologous T-cell therapy engineered with hepatitis B virus (HBV)-specific T-cell receptors – SCG101 – was highlighted in a late-breaking session at the European Association for the Study of the Liver Congress 2024, held in Milan, Italy.
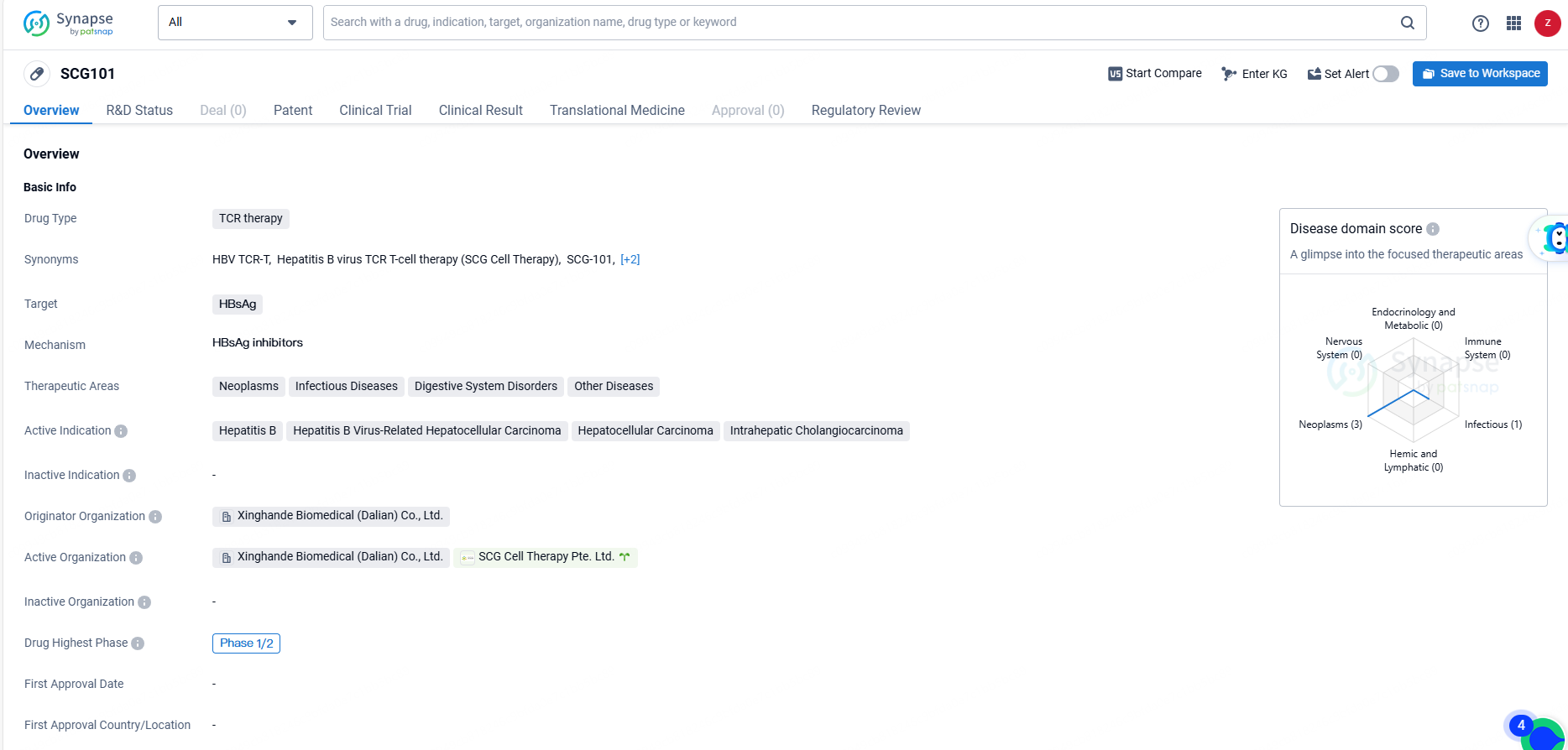
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The talk was chosen to be part of the Best of EASL Congress Wrap-Up summary, encapsulating the most outstanding highlights, emerging trends, and significant contributions to the EASL agenda.
The safety evaluation indicated that SCG101 was generally well tolerated, with no incidents of serious adverse events or immune effector cell-associated neurotoxicity syndrome. The predominant SCG101-related adverse events were transient liver enzyme elevation, cytokine release syndrome, and fever, which align with SCG101’s action in clearing cells expressing HBsAg.
“We are thrilled that our pivotal data was showcased at the EASL Wrap-up session, demonstrating the convincing dual antiviral and antitumor profile of SCG101 with sustained HBsAg reduction and prolonged progression-free and overall survival in HBV-HCC patients,” stated Christy Ma, CEO of SCG Cell Therapy.
“These findings underscore the robustness of our GianTCRTM platform in generating promising TCR-based therapeutic candidates across various therapeutic areas, especially for infectious diseases and related cancers. We eagerly anticipate further exploration of SCG101 and other TCR candidates to deliver beneficial outcomes for patients with unmet medical needs.” Christy Ma continued.
SCG101, an autologous T-cell receptor T cell therapy, is an investigational product aimed at a specific epitope of hepatitis B surface antigen. Leveraging SCG’s proprietary GianTTM technology, high affinity and high avidity natural TCRs can be identified against intracellular antigens presented via the major histocompatibility complex in solid tumors.
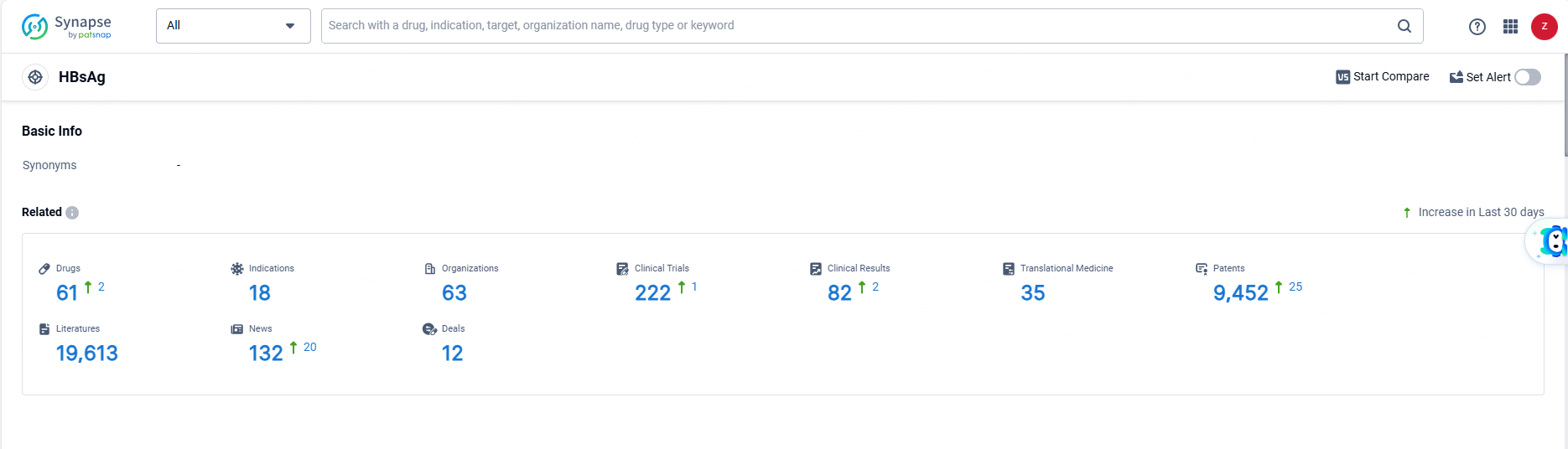
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 13, 2024, there are 61 investigational drugs for the HBsAg targets, including 18 indications, 63 R&D institutions involved, with related clinical trials reaching 222, and as many as 9452 patents.
SCG101 targets HBsAg and is intended for the treatment of various conditions, including neoplasms, infectious diseases, digestive system disorders, and other diseases. The development of SCG101 represents a significant advancement in the field of biomedicine, particularly in the treatment of hepatitis B and related conditions such as hepatocellular carcinoma and intrahepatic cholangiocarcinoma. The drug's orphan drug status also highlights its potential to address unmet medical needs in rare diseases.