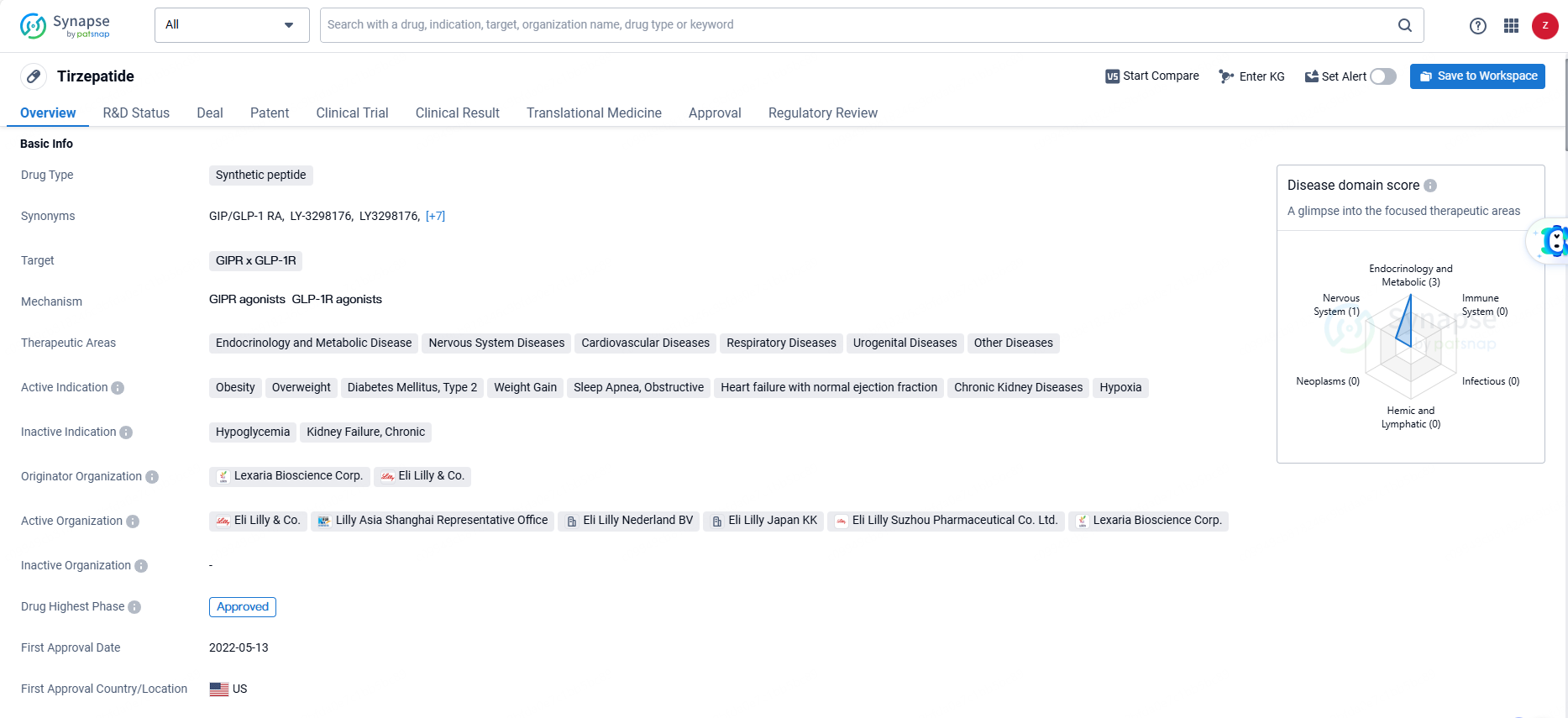
Lilly's Tirzepatide: Over 50% See Fibrosis Improvement and MASH Resolution After 52 Weeks
Eli Lilly and Company has revealed comprehensive findings from SYNERGY-NASH, a phase 2 clinical trial involving 190 participants, regardless of type 2 diabetes status. This study assessed the investigational application of tirzepatide in adults diagnosed with biopsy-confirmed metabolic dysfunction-associated steatohepatitis (NASH) having stage 2 or 3 fibrosis.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The efficacy estimand demonstrated that 51.8%, 62.8%, and 73.3% of participants taking 5 mg, 10 mg, and 15 mg doses, respectively, achieved the resolution of MASH without any fibrosis worsening on liver histology at 52 weeks. This was in contrast to 13.2% of participants on placebo, thereby meeting the primary endpoint of the study. These findings were presented at the European Association for the Study of the Liver Congress 2024 and were concurrently published in The New England Journal of Medicine.
For a secondary endpoint, the efficacy estimand indicated that 59.1%, 53.3%, and 54.2% of participants taking 5 mg, 10 mg, and 15 mg, respectively, showed a 1-stage or greater improvement in fibrosis without any worsening of MASH, compared to 32.8% of participants on placebo.
Additional secondary endpoints assessed suggested that tirzepatide was linked to improvements in body weight, blood markers of liver injury, and biomarkers related to liver fat, inflammation, and fibrosis. Despite the phase 2 study not being designed to definitively establish that tirzepatide improves fibrosis, the results indicated a potential for a meaningful clinical effect across all dosages.
“MASH is the second leading cause of liver transplantation in the U.S., underlining the necessity for new therapeutic approaches,” stated Rohit Loomba, MD, MHSc, chief of the division of gastroenterology and hepatology at University of California San Diego School of Medicine. “The study's importance is highlighted by the urgent requirement for treatments that can slow disease progression and potentially reduce severe health complications.”
The overall safety profile of tirzepatide in the SYNERGY-NASH trial was comparable to that observed in the SURMOUNT and SURPASS trials. The most frequently reported adverse events in SYNERGY-NASH were gastrointestinal in nature and were generally mild to moderate in severity.
“Lilly is very pleased with the extent of MASH resolution observed in the SYNERGY-NASH study, and we are optimistic about the fibrosis improvements noted,” said Jeff Emmick, MD, PhD, senior vice president of product development at Lilly. “MASH is projected to affect more than 19 million adults in the U.S. by 2039, and based on these findings, we believe tirzepatide might be an effective treatment for individuals suffering from this disease.”
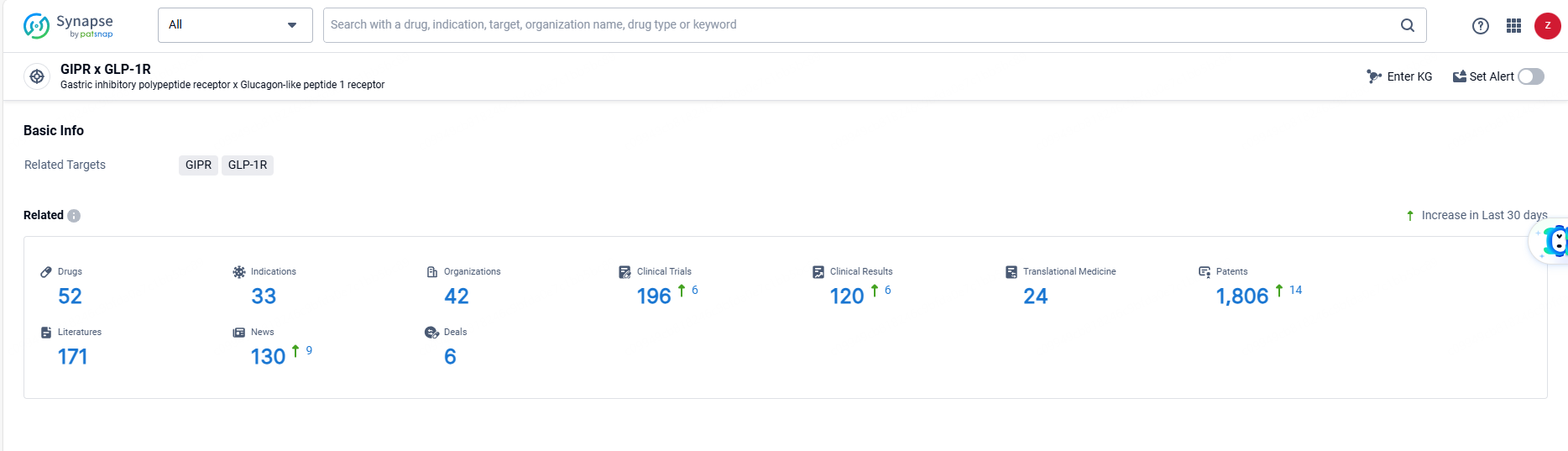
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 13, 2024, there are 52 investigational drugs for the GIPR and GLP-1R target, including 33 indications, 42 R&D institutions involved, with related clinical trials reaching 196, and as many as 1806 patents.
Tirzepatide is a synthetic peptide drug that targets the GIPR and GLP-1R receptors. It is indicated for a wide range of therapeutic areas including endocrinology and metabolic disease, nervous system diseases, cardiovascular diseases, respiratory diseases, urogenital diseases, and other diseases. Tirzepatide is a synthetic peptide drug that targets multiple receptors and has been approved for various therapeutic areas, particularly in the treatment of metabolic and endocrine disorders. Its approval in the United States and China, as well as its fast track and priority review status, highlight its potential to address critical medical conditions.