How to find the chemical modification of tivanisiran?
Tivanisiran is an innovative small interfering RNA (siRNA) therapeutic agent developed by Sylentis, a Spanish biopharmaceutical company specializing in RNAi technology. This novel drug targets the transient receptor potential cation channel subfamily V member 1 (TRPV1) and is being investigated for the treatment of dry eye disease (DED). As a small molecule RNAi therapy, tivanisiran represents a cutting-edge approach in the field of ophthalmology, aiming to provide relief for patients suffering from this chronic and often debilitating condition.
Summary of Research Progress of tivanisiran
The research progress of tivanisiran has been marked by promising results in clinical trials and a growing understanding of its mechanism of action. At its core, tivanisiran works by silencing the TRPV1 gene, which plays a crucial role in pain sensation and inflammation associated with dry eye disease. By targeting this specific receptor, tivanisiran aims to reduce the symptoms of DED and improve ocular surface health.
The global listing situation for tivanisiran is still in development, as the drug has not yet received regulatory approval in any country. However, Sylentis has made significant strides in advancing the drug through clinical trials. The most notable progress came from the Phase III HELIX trial, which demonstrated improvements in central corneal staining reduction (p=0.035). This positive outcome suggests that tivanisiran has the potential to become a valuable treatment option for DED patients in the future.
In terms of global competition, tivanisiran enters a market with several established treatments for dry eye disease, including cyclosporine and lifitegrast. However, the scarcity of long-term marketed drugs for DED represents an opportunity for tivanisiran to address an unmet medical need. The innovative RNAi approach of tivanisiran sets it apart from traditional therapies and may offer advantages in terms of specificity and potentially fewer side effects.
Clinical research status for tivanisiran has progressed through various phases. The Phase III HELIX trial has been a key milestone in its development, providing evidence of efficacy in reducing central corneal staining. This trial's results are crucial for advancing tivanisiran towards potential regulatory approval. Additionally, ongoing research continues to evaluate the long-term safety and efficacy of the drug in diverse patient populations.
Sequence Information and Characteristics of tivanisiran
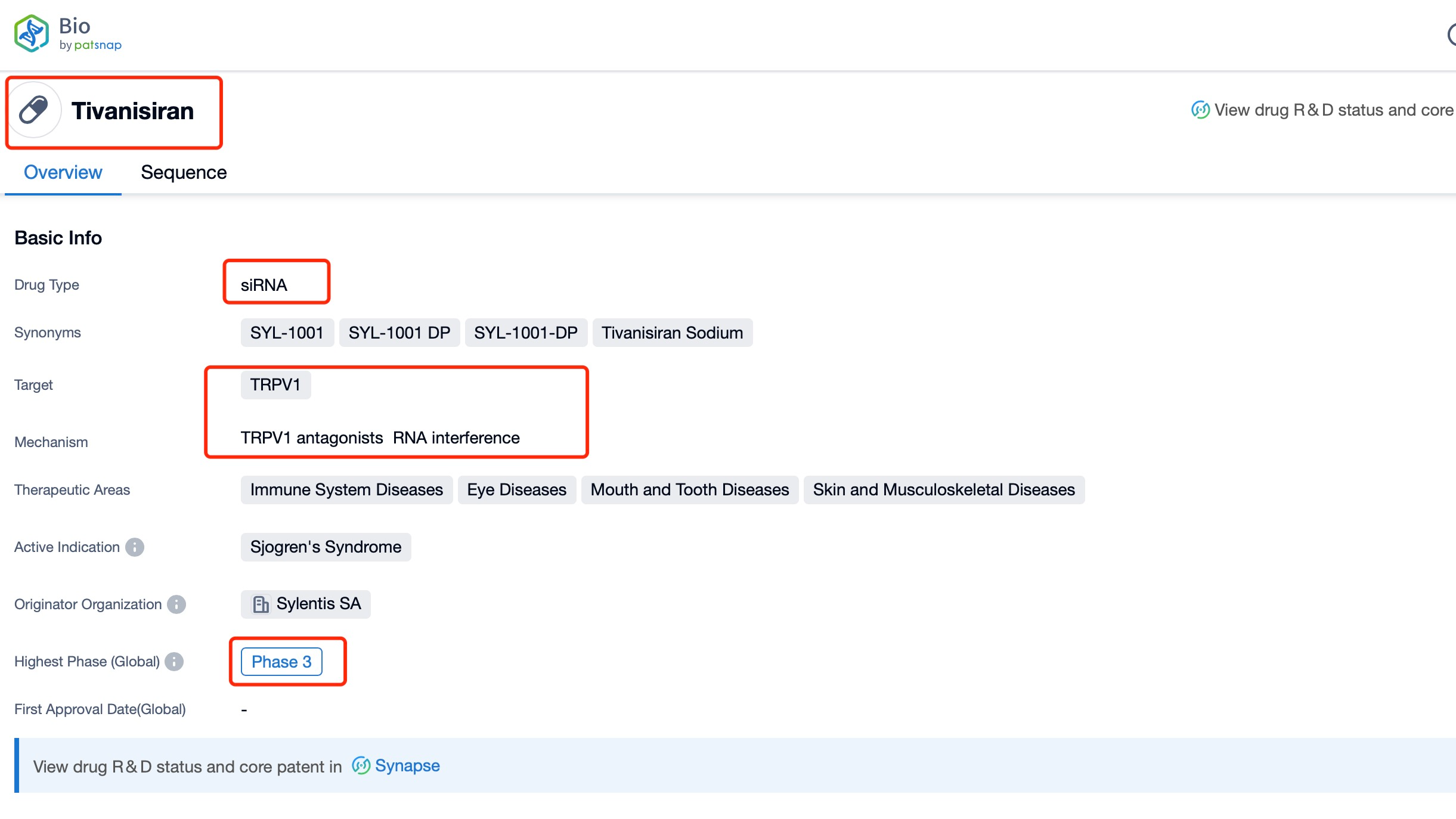
Understanding the sequence information and characteristics of tivanisiran is fundamental to grasping its unique properties as an RNAi therapeutic. The exact sequence details are provided by PatSnap Bio. Tivanisiran, as an siRNA molecule, consists of a short double-stranded RNA sequence specifically targeting TRPV1 mRNA. This specificity is a hallmark of RNAi technology, enabling precise regulation of gene expression.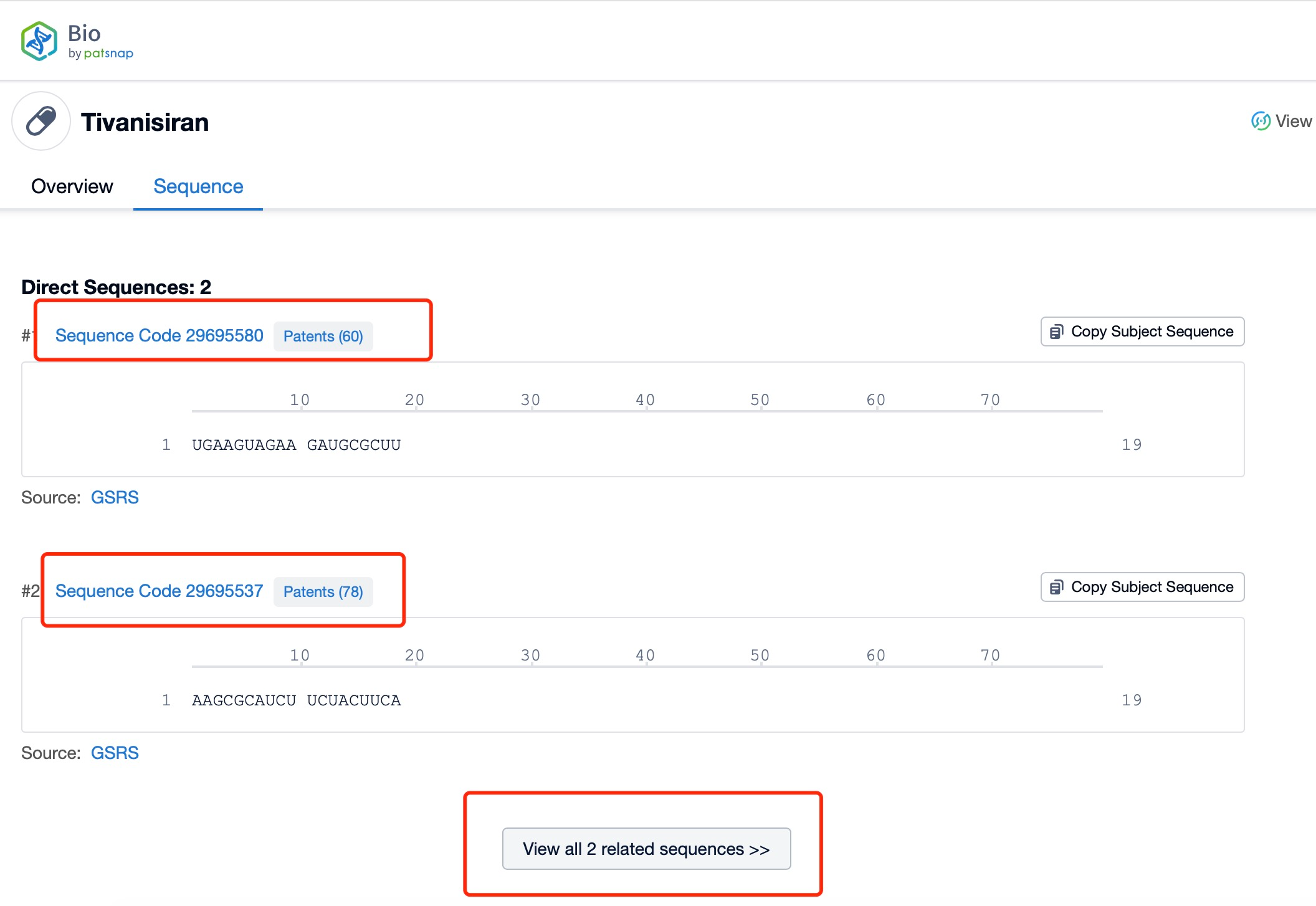
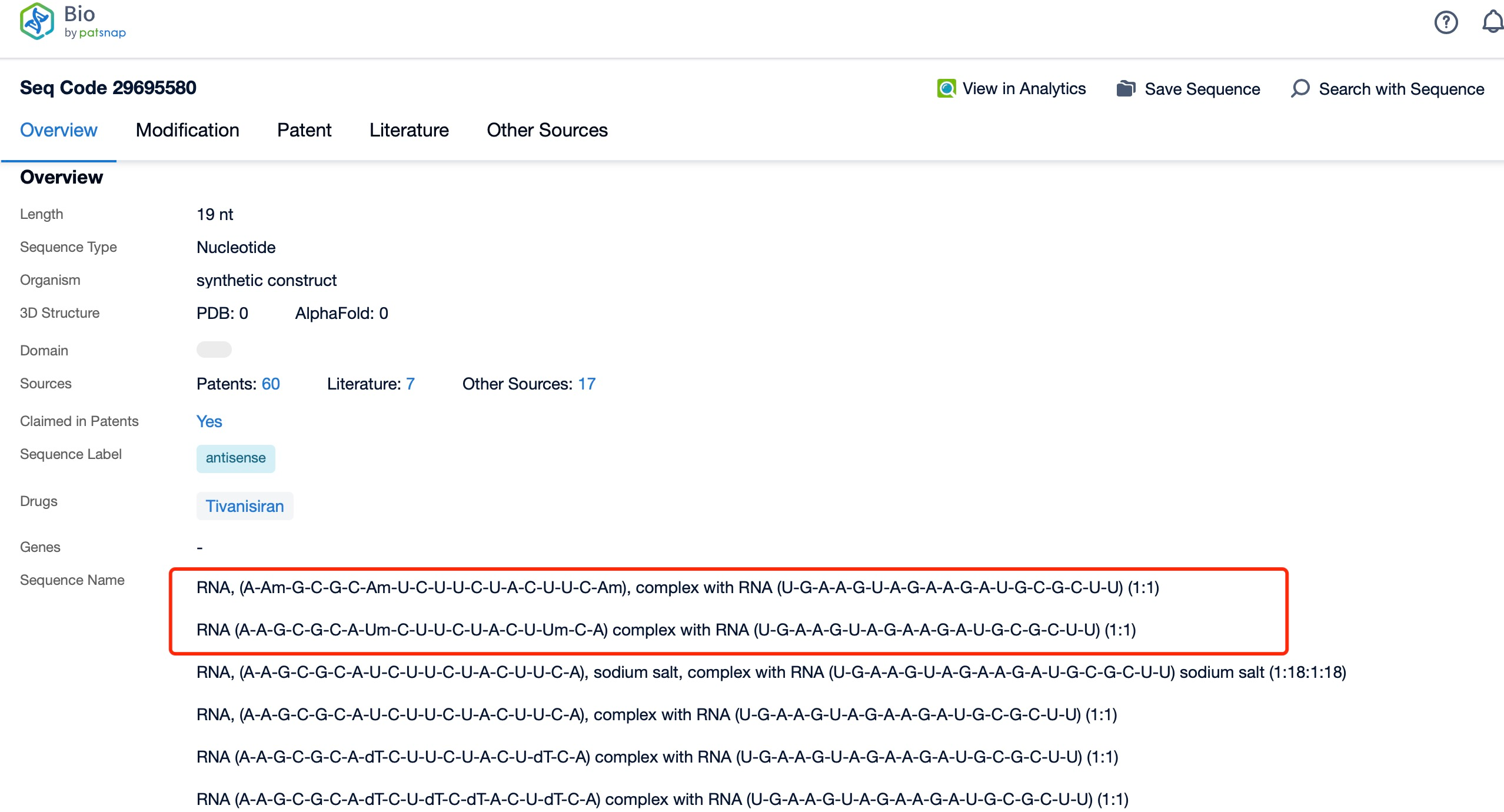
The sequence of tivanisiran is composed of 19 nucleotides, which is typical for siRNA molecules. This length is optimized to ensure efficient incorporation into the RNA-induced silencing complex (RISC) while maintaining target specificity. The sequence would be complementary to a portion of the TRPV1 mRNA, allowing for precise binding and subsequent degradation of the target transcript.
Chemical Modification and Action of tivanisiran
One of the unique characteristics of tivanisiran is its ability to penetrate ocular tissues effectively. This is crucial for an ophthalmic medication, as the eye presents significant barriers to drug delivery. The designers of tivanisiran incorporated chemical modifications to enhance its stability and cellular uptake in ocular tissues. These modifications include the use of phosphorothioate linkages and 2'-O-methyl nucleotides, which are common strategies to improve the pharmacokinetic properties of siRNA drugs.
Another important characteristic of tivanisiran is its specificity for the TRPV1 target. This specificity is determined by the precise sequence complementarity between the siRNA and its target mRNA. By focusing on TRPV1, tivanisiran aims to modulate a key pathway involved in dry eye disease pathogenesis without affecting other cellular processes, potentially leading to a more favorable side effect profile compared to broader-acting drugs.
The chemical modification and action of tivanisiran are critical aspects of its therapeutic potential. As an siRNA molecule, tivanisiran undergoes several chemical modifications to enhance its drug-like properties. These modifications are essential for overcoming the challenges associated with delivering RNA-based therapeutics, particularly to the eye.
One common modification in siRNA drugs is the incorporation of 2'-O-methyl or 2'-fluoro groups on the ribose sugars of the RNA backbone. These changes increase the molecule's resistance to degradation by endogenous nucleases, thereby extending its half-life in biological systems. Additionally, the phosphodiester bonds between nucleotides may be replaced with phosphorothioate linkages at strategic positions. This modification not only improves nuclease resistance but also enhances cellular uptake and distribution of the siRNA.
The chemical structure of tivanisiran would also include modifications to reduce off-target effects and minimize immune stimulation. This could involve careful selection of the seed region sequence and the incorporation of unlocked nucleic acid (UNA) monomers or other nucleotide analogs that fine-tune the thermodynamic properties of the siRNA duplex.
The action of tivanisiran begins when it enters target cells in the ocular tissues. Once inside the cell, the siRNA is recognized by the RISC complex. The guide strand of tivanisiran is incorporated into RISC, while the passenger strand is discarded. The RISC-associated guide strand then directs the complex to complementary TRPV1 mRNA sequences. Upon binding, the RISC complex cleaves the target mRNA, leading to its degradation and subsequent reduction in TRPV1 protein expression.
This mechanism of action is highly specific and potent, allowing for sustained downregulation of TRPV1 with relatively low doses of the drug. The reduced expression of TRPV1 is thought to alleviate symptoms of dry eye disease by decreasing pain sensation and inflammatory responses in the ocular surface.
Summary and Prospect
In summary, tivanisiran represents a promising advancement in the treatment of dry eye disease. As an siRNA therapeutic targeting TRPV1, it offers a novel approach to managing this common ocular condition. The positive results from the Phase III HELIX trial, demonstrating improvements in central corneal staining, highlight its potential efficacy. The specificity of tivanisiran for TRPV1 and its RNAi-based mechanism of action set it apart from traditional treatments and may offer advantages in terms of targeted therapy with potentially fewer systemic side effects.
The development of tivanisiran also reflects the growing importance of RNAi therapeutics in ophthalmology and medicine as a whole. As one of the most advanced experimental strategies in the pipeline for DED, alongside other innovative approaches like IL-1R antagonists and SkQ1, tivanisiran is at the forefront of efforts to address the limitations of current DED treatments.
Looking to the future, the prospects for tivanisiran appear promising. If approved, it could provide a much-needed alternative for patients who have not responded well to existing therapies. The success of tivanisiran could also pave the way for further development of RNAi-based treatments in ophthalmology and other medical fields.
However, several challenges remain. Long-term safety data will be crucial for regulatory approval and widespread adoption. Additionally, optimizing the delivery and dosing regimen for chronic use in DED patients will be important for maximizing therapeutic benefits while minimizing potential side effects.
Future research directions for tivanisiran may include exploring its efficacy in combination with other DED treatments, investigating its potential in related ocular surface disorders, and further refining its chemical structure to enhance its pharmacokinetic properties. As the understanding of dry eye disease pathophysiology continues to evolve, tivanisiran's targeted approach may also lead to new insights into the role of TRPV1 in ocular surface health.
In conclusion, tivanisiran represents a significant step forward in the quest for more effective and targeted treatments for dry eye disease. Its progress through clinical trials and innovative mechanism of action makes it a promising candidate to watch in the field of ophthalmology. As research continues and potential regulatory approvals approach, tivanisiran may soon offer new hope to millions of patients suffering from this chronic and often underserved condition.
How to find the chemical modification of all siRNAs?
In Patsnap Bio, you can find the sequence and latest research and development advances of all siRNAs.
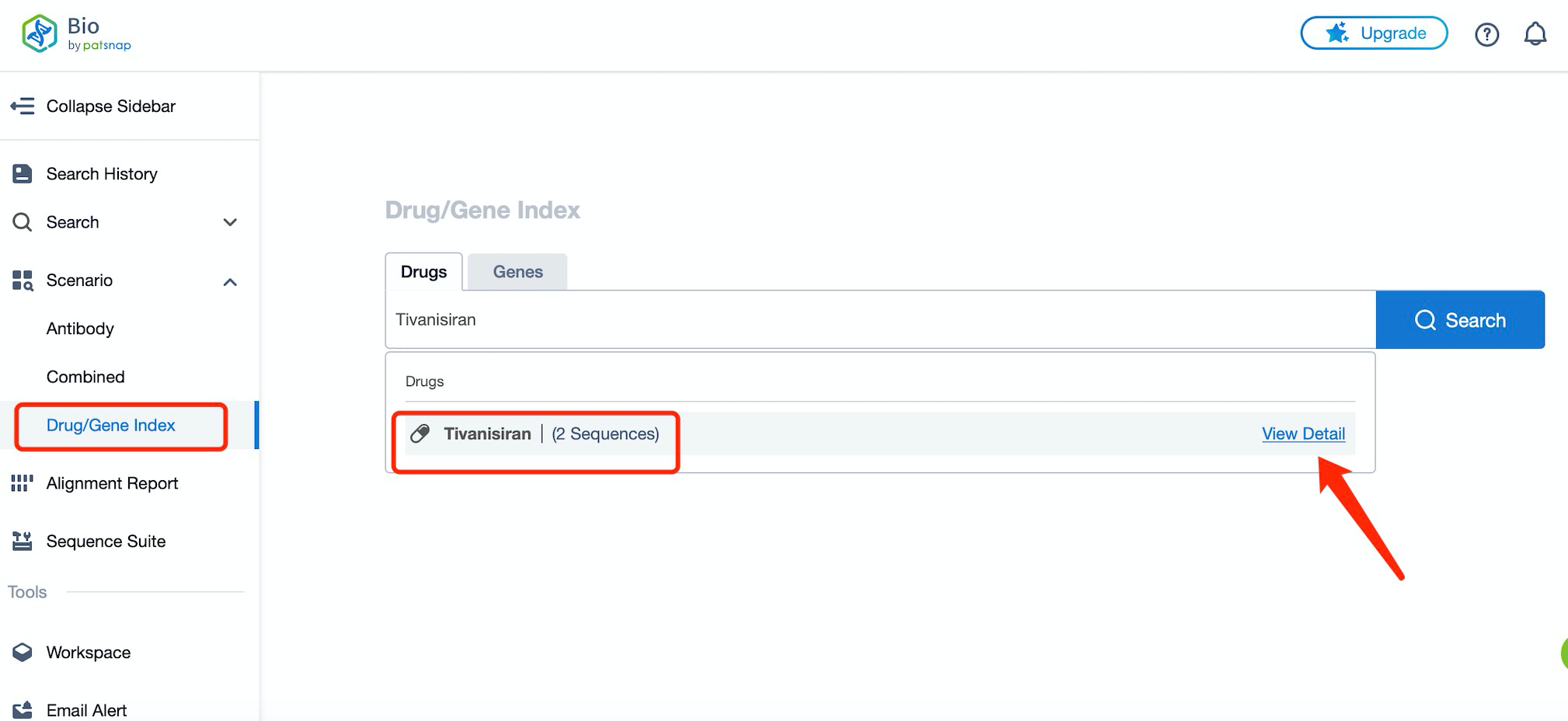
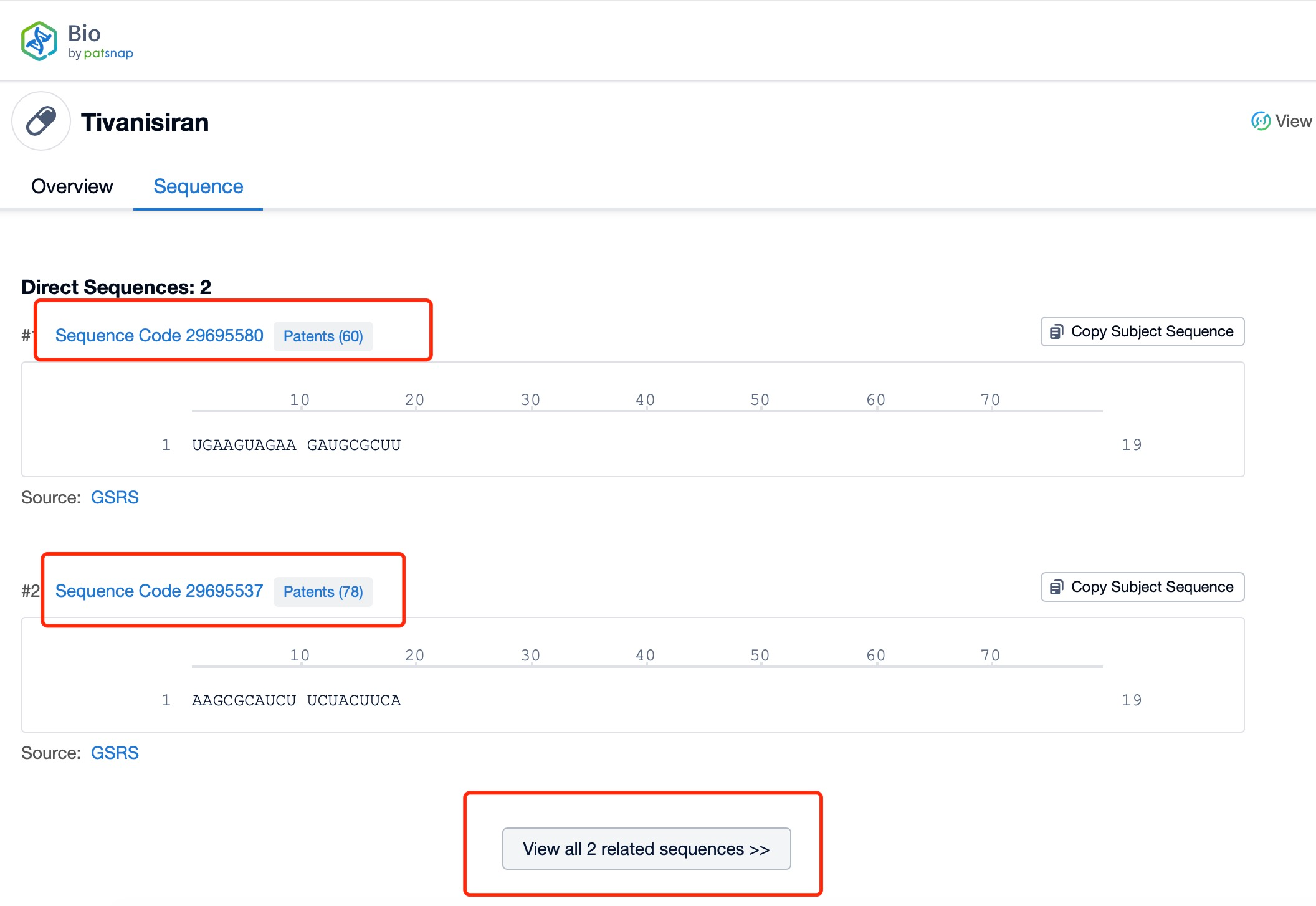
Taking tivanisiran as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' tivanisiran ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of tivanisiran.
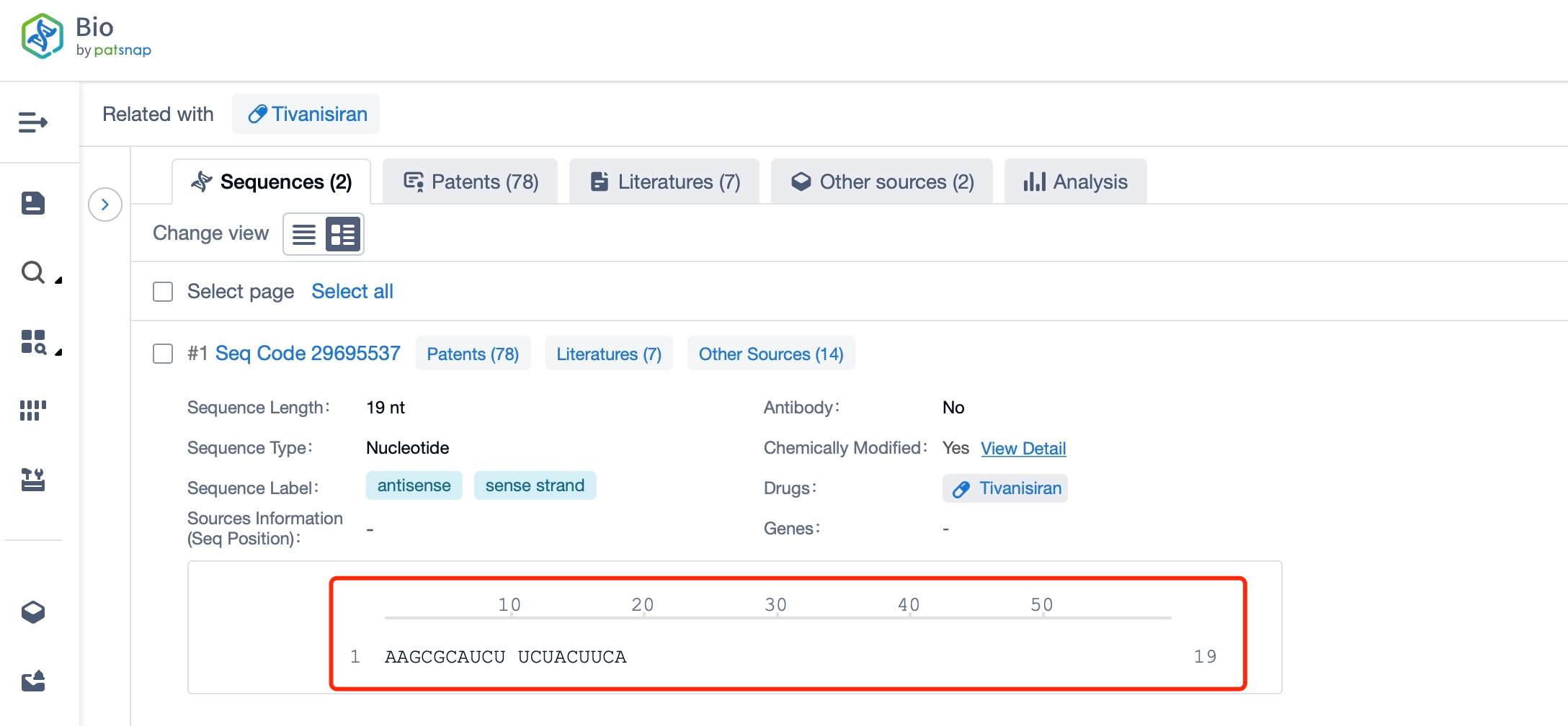
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
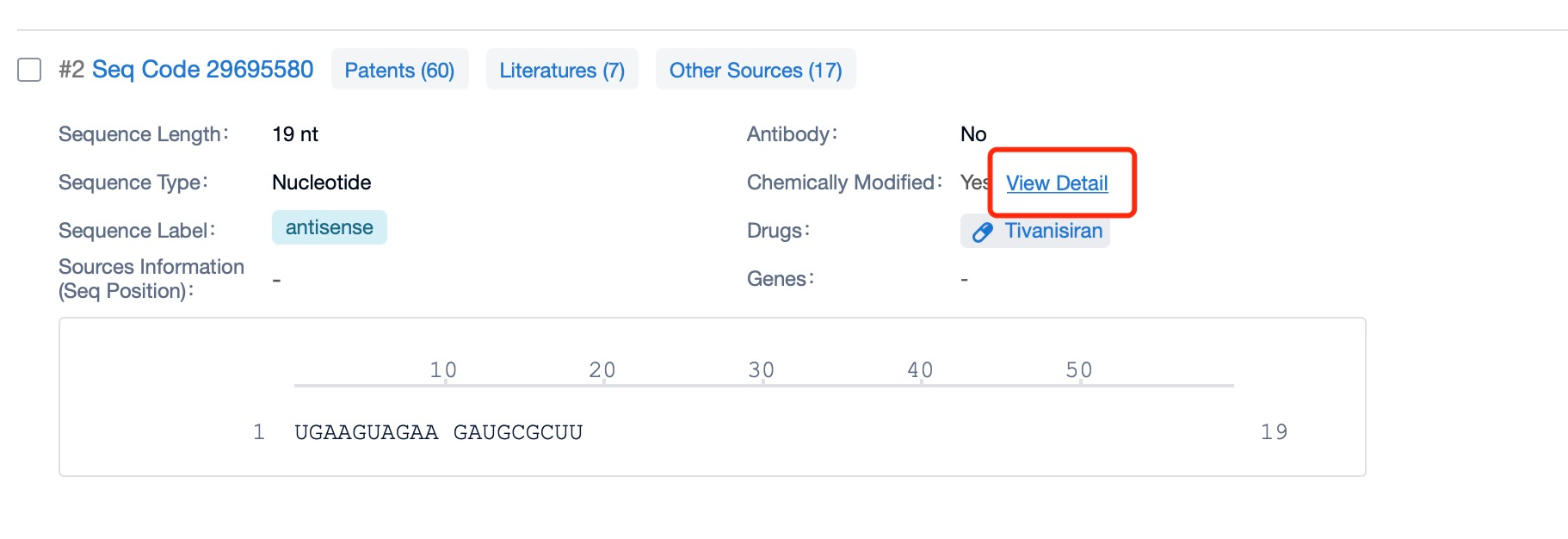
Clicking on the sequence name will provide you with all the basic information of that sequence.
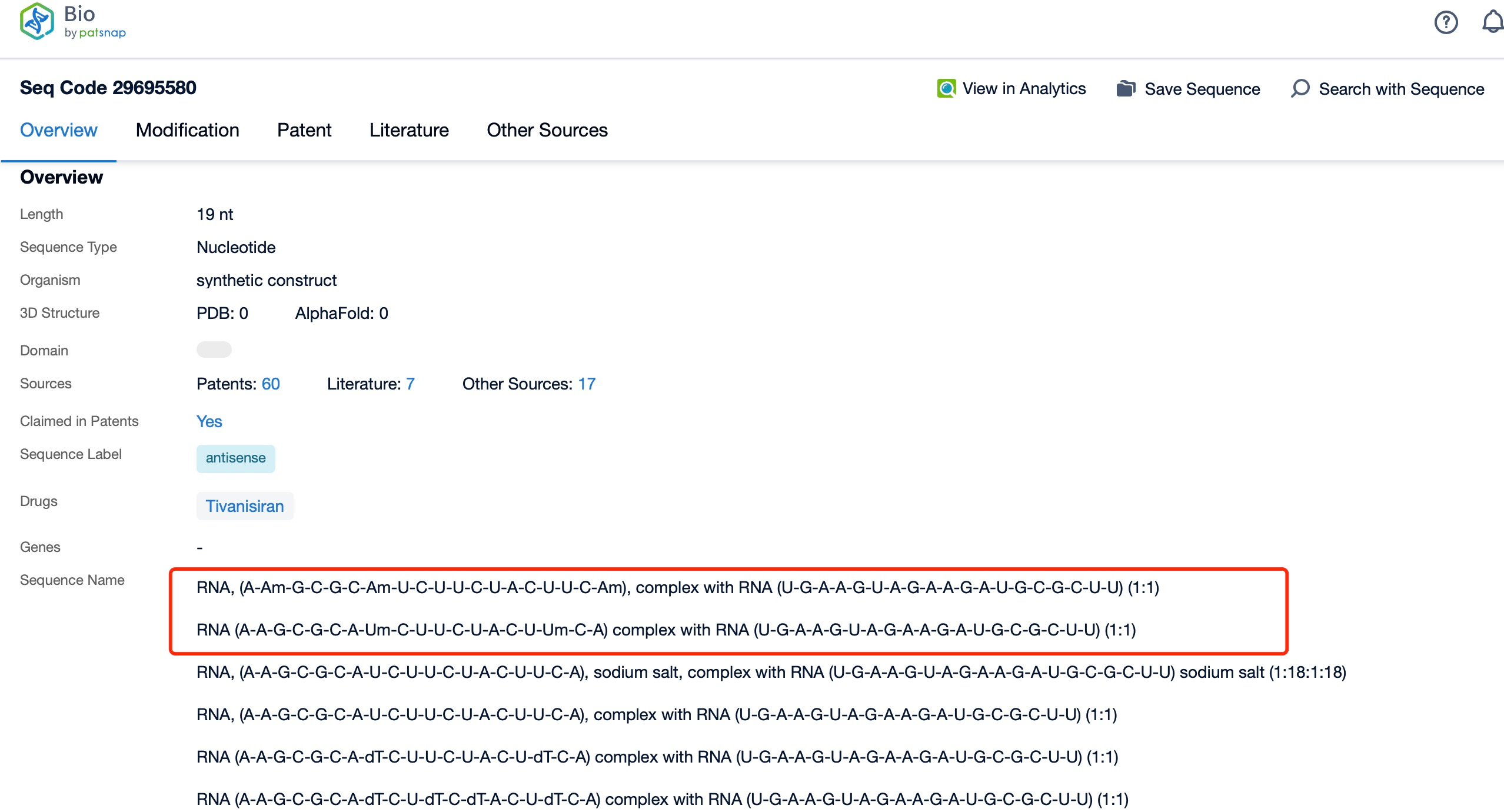
Additionally, a visual diagram of the sequence's chemical modifications is available for immediate access.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.