FDA Grants Priority Review for argenx's VYVGART Hytrulo in Chronic Inflammatory Demyelinating Polyneuropathy
Argenx SE has disclosed that its extended Biologics License Application for VYVGART Hytrulo, aimed at treating chronic inflammatory demyelinating polyneuropathy, has been officially received by the U.S. Food and Drug Administration for an expedited evaluation. The submission has met the criteria for a Prescription Drug User Fee Act (PDUFA) target decision date.
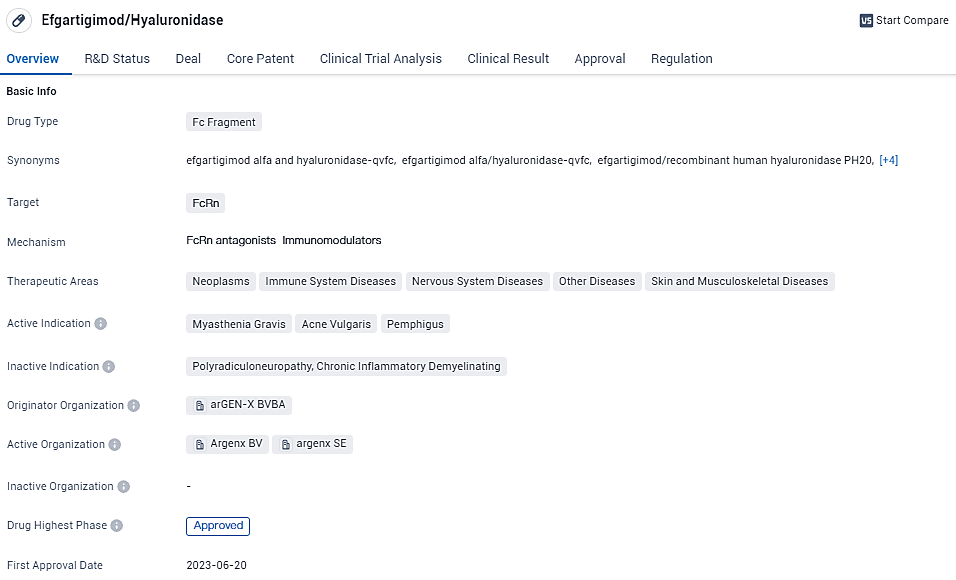
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"Luc Truyen, the Chief Medical Officer at argenx, expressed enthusiasm over the recent update, stating, 'This announcement marks a significant leap towards making the groundbreaking VYVGART Hytrulo therapy accessible to individuals living with CIDP.' The FDA's recognition of the sBLA is a crucial achievement in our quest to introduce innovative therapies for rare autoimmune disorders, offering hope to those severely affected by this incapacitating illness."
The sBLA gains robust backing from the ADHERE trial outcomes, the most extensive CIDP clinical research conducted to date. This study focused on the safety and therapeutic outcomes of VYVGART Hytrulo when administered via subcutaneous injections in the adult CIDP population. Results indicated the primary goal of the trial was achieved, showcasing a 61% reduction in the recurrence risk when using VYVGART Hytrulo compared to a placebo.
During the preliminary open-label Phase A of the trial, clinical improvement was evident in 67% of participants treated with VYVGART Hytrulo. Further underlining the significance of FcRn blocker mechanism, the findings confirmed the central role of IgG autoantibodies in the pathogenesis of CIDP.
In terms of tolerability, VYVGART Hytrulo demonstrated a safety profile aligned with previous studies and the established profile of the intravenous VYVGART® formulation. Post ADHERE trial, an overwhelming majority of eligible subjects, 99% (226 out of 228), opted to proceed with the ADHERE-+ open-label extension study.
VYVGART Hytrulo comprises a subcutaneous blend, including efgartigimod alfa, which is an IgG1 human antibody segment already marketed as VYVGART® for intravenous applications, combined with Halozyme's ENHANZE® drug delivery technology, which incorporates recombinant human hyaluronidase PH20 to aid the subcutaneous administration of biological treatments.
When VYVGART Hytrulo connects with the neonatal Fc receptor (FcRn), it triggers a reduction in circulating IgG. This compound stands out as the pioneer FcRn inhibitor that is dispensed through a subcutaneous injection.
Within the United States, VYVGART Hytrulo is the official designation for the subcutaneous mixture of efgartigimod alfa and recombinant human hyaluronidase PH20. Post-approval in various other locations, it could be introduced under alternative trade names.
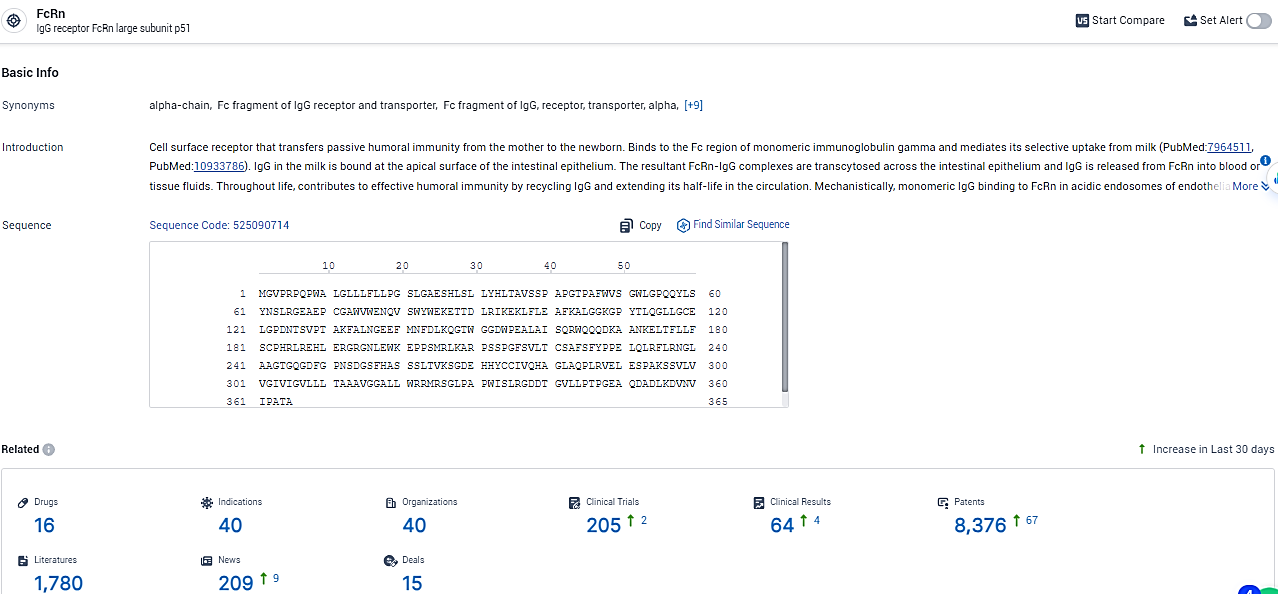
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 23, 2024, there are 16 investigational drugs for the FcRn target, including 40 indications, 40 R&D institutions involved, with related clinical trials reaching 205, and as many as 8376 patents.
Efgartigimod/Hyaluronidase is an Fc Fragment drug that targets FcRn and has shown potential in treating various therapeutic areas, including neoplasms, immune system diseases, nervous system diseases, other diseases, and skin and musculoskeletal diseases. Developed by arGEN-X BVBA, the drug has received global approval and is awaiting approval in China. Its first approval is expected in June 2023 in the United States, and it is regulated as an orphan drug.