Exploring Reteplase's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Reteplase's R&D Progress
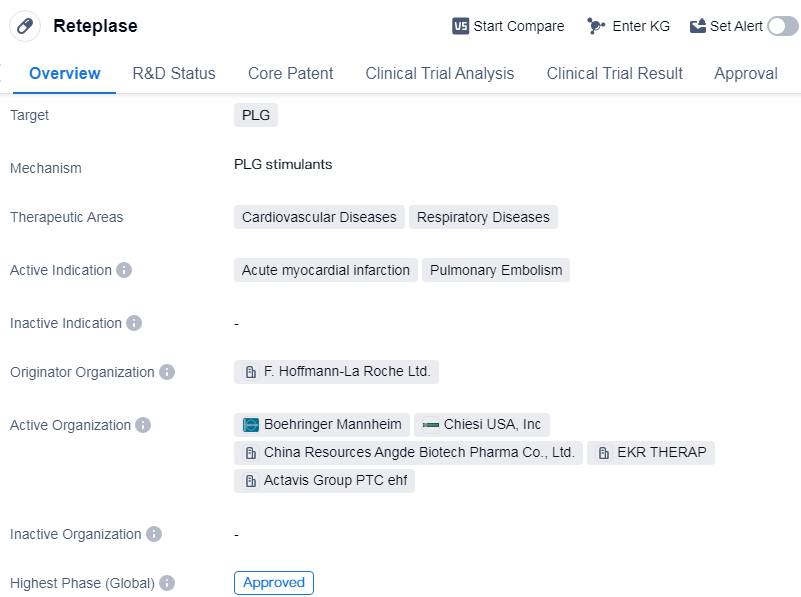
Reteplase is an enzyme-based drug that targets plasminogen (PLG) and is primarily used in the treatment of cardiovascular and respiratory diseases. It is indicated for acute myocardial infarction (heart attack) and pulmonary embolism (blockage of the lung arteries).
The drug was developed by F. Hoffmann-La Roche Ltd., a renowned pharmaceutical company. Reteplase has undergone extensive clinical trials and has reached the highest phase of approval globally. It was first approved in the European Union in August 1996, making it available for use in the treatment of the specified conditions.
Reteplase's mechanism of action involves its ability to activate plasminogen, which in turn leads to the breakdown of blood clots. This property makes it particularly effective in treating acute myocardial infarction and pulmonary embolism, where the prompt dissolution of blood clots is crucial.
In terms of its therapeutic areas, Reteplase is primarily used in the treatment of cardiovascular diseases, specifically acute myocardial infarction. It is also indicated for respiratory diseases, particularly pulmonary embolism. These conditions are serious and potentially life-threatening, making Reteplase an important drug in the field of biomedicine.
While Reteplase has reached the highest phase of approval globally, it is currently in phase 2 of development in China. This suggests that the drug is still undergoing further evaluation and testing in the Chinese market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Reteplase: PLG stimulants
From a general understanding, PLG stimulants refer to substances or compounds that have the ability to stimulate or enhance the activity of the protein PLG (plasminogen). PLG is a precursor protein that plays a crucial role in the fibrinolytic system, which is responsible for breaking down blood clots. By stimulating PLG, these substances can potentially promote the breakdown of blood clots more efficiently.
From a biomedical perspective, PLG stimulants can be of interest in the context of thrombolytic therapy, which aims to dissolve blood clots in various medical conditions such as heart attacks, strokes, or deep vein thrombosis. By enhancing the activity of PLG, these stimulants could potentially improve the effectiveness of thrombolytic treatments and contribute to better patient outcomes.
It is important to note that the specific substances or compounds referred to as PLG stimulants may vary, and further context would be needed to provide more specific information about their nature, mechanisms of action, or potential applications.
Drug Target R&D Trends for Reteplase
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 82 PLG drugs worldwide, from 116 organizations, covering 58 indications, and conducting 1730 clinical trials.
The current competitive landscape of the target PLG indicates that companies like Roche Holding AG, bioMérieux SA, Chandra Bhagat Pharma Ltd., Shanghai Fosun High Technology (Group) Co., Ltd., and Bharat Serums & Vaccines Ltd. are growing rapidly. These companies have made significant progress in R&D, with multiple drugs approved under the target PLG. The highest stage of development on this target is the approved phase.
The indications with the highest number of approved drugs under the target PLG include myocardial infarction, pulmonary embolism, thromboembolism, and thrombosis.
The drug types progressing most rapidly under the target PLG are enzyme, biosimilar, and small molecule drug. The presence of biosimilars indicates intense competition around the innovative drugs, with companies like Roche Holding AG, C.H. Boehringer Sohn AG & Co. KG, and Pfizer Inc. involved in their development.
China and India are the countries that have shown the fastest development under the target PLG. China, in particular, has made significant progress, with the highest number of approved drugs. This highlights the growing importance of these countries in the pharmaceutical industry.
Overall, the target PLG presents a competitive landscape with companies making significant progress in R&D and multiple drugs approved for various indications. The future development of the target PLG is likely to see continued growth in China and India, along with intense competition in the biosimilar market.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Reteplase is an enzyme-based drug developed by F. Hoffmann-La Roche Ltd. It targets plasminogen and is primarily used in the treatment of cardiovascular and respiratory diseases. Its active indications include acute myocardial infarction and pulmonary embolism. Reteplase has been approved for use in the European Union since 1996 and has reached the highest phase of approval globally. However, it is currently in phase 2 of development in China.