What Does Tisagenlecleucel Do?
Tisagenlecleucel, marketed under the brand name Kymriah, is a pioneering immunotherapy medication developed by Novartis. It is a chimeric antigen receptor T cell (CAR-T) therapy, specifically targeting the CD19 protein found on the surface of certain B-cell lymphocytes associated with leukemia.
Tisagenlecleucel is approved for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) in patients up to 25 years old, and for adult patients with relapsed or refractory large B-cell lymphoma (DLBCL) after at least two other treatments have failed. The drug is administered under a special program that requires patient registration and a thorough understanding of the treatment's risks and benefits.
Mechanism of Action of Tisagenlecleucel
Tisagenlecleucel's mechanism of action involves the use of the patient's own T-cells, a type of white blood cell crucial for immune responses. The process begins with leukapheresis, where T-cells are collected from the patient's blood. These cells are then genetically modified in a laboratory to produce a protein that enables them to recognize and attach to CD19 on B-cell surfaces. Once infused back into the patient, these modified T-cells multiply and target the cancerous B-cells for destruction, offering a personalized and targeted approach to fighting leukemia.
How to Use Tisagenlecleucel
Tisagenlecleucel is not a traditional drug that can be taken orally; it is a one-time infusion therapy. The treatment is administered only at authorized hospitals or clinics by specialized healthcare professionals. Before the infusion, patients undergo a preparatory chemotherapy treatment and may be given medications to prevent serious side effects or allergic reactions. The actual infusion process involves injecting the modified T-cells into a vein through an IV. After treatment, patients are advised to stay near the treatment facility for at least four weeks to allow for close monitoring.
Side Effects of Tisagenlecleucel
Tisagenlecleucel can cause a range of side effects, including cytokine release syndrome (CRS), which is a potentially life-threatening condition characterized by symptoms such as fever, chills, trouble breathing, body aches, vomiting, diarrhea, and feeling light-headed. Other severe side effects may involve life-threatening nerve problems, such as speech issues, confusion, memory problems, and seizures. Common side effects can include nausea, vomiting, diarrhea, loss of appetite, fever, headache, confusion, tiredness, bleeding, and fast heartbeats.
Drug Interactions with Tisagenlecleucel
Tisagenlecleucel may interact with other medications, including prescription drugs, over-the-counter medicines, vitamins, and herbal products. It is essential to inform healthcare providers of all current medications before starting treatment with tisagenlecleucel. Additionally, patients should avoid receiving live vaccines before and after treatment, as the therapy can suppress the immune system. Tisagenlecleucel can also cause a false positive HIV test, so it is crucial to inform any treating doctors of the treatment to avoid diagnostic errors.
In conclusion, Tisagenlecleucel represents a breakthrough in personalized cancer treatment, offering new hope for patients with certain types of leukemia and lymphoma. However, its use requires careful consideration of potential side effects and drug interactions, highlighting the importance of close collaboration between patients and healthcare providers throughout the treatment process.
How to obtain the latest development progress of all drugs?
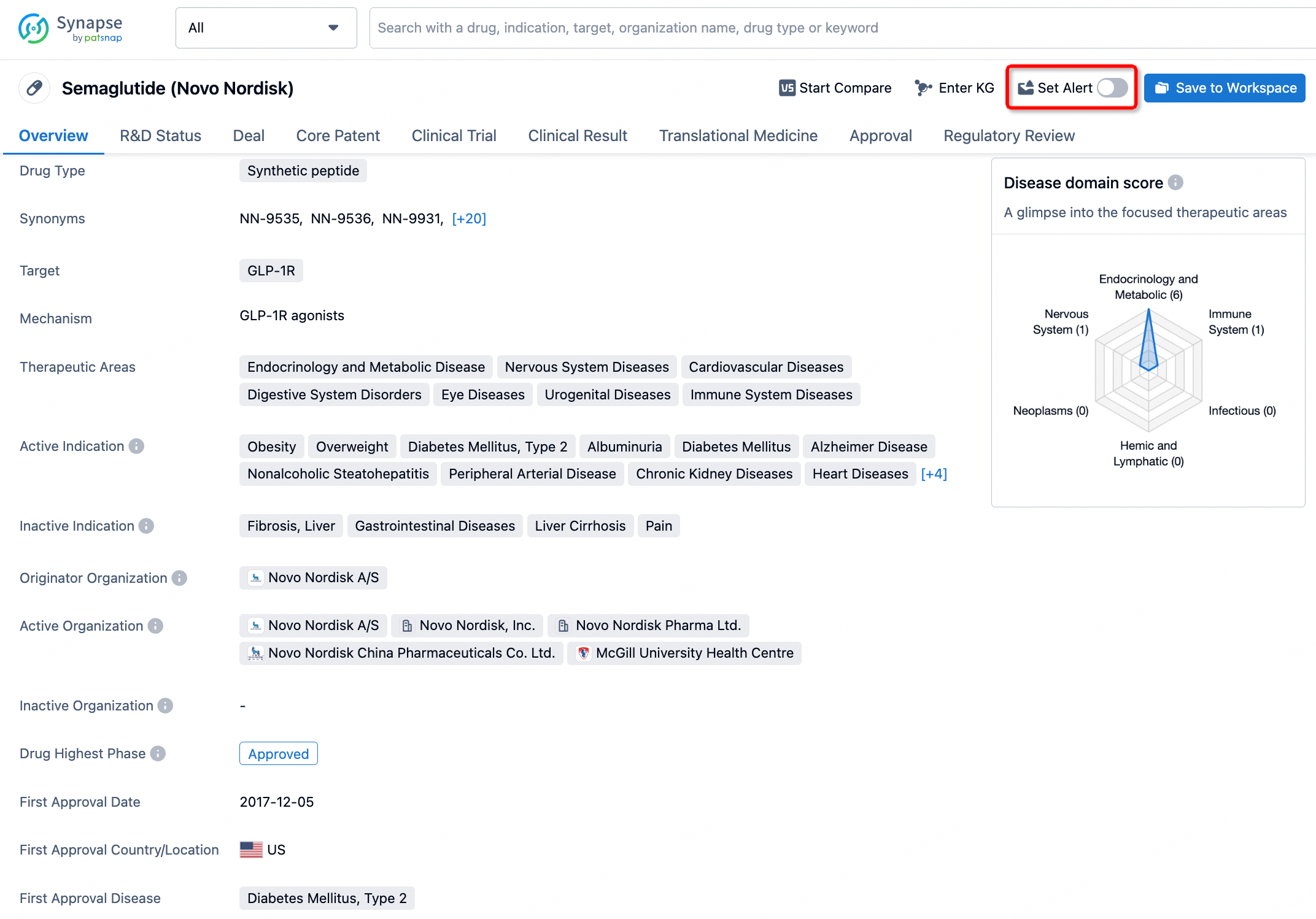
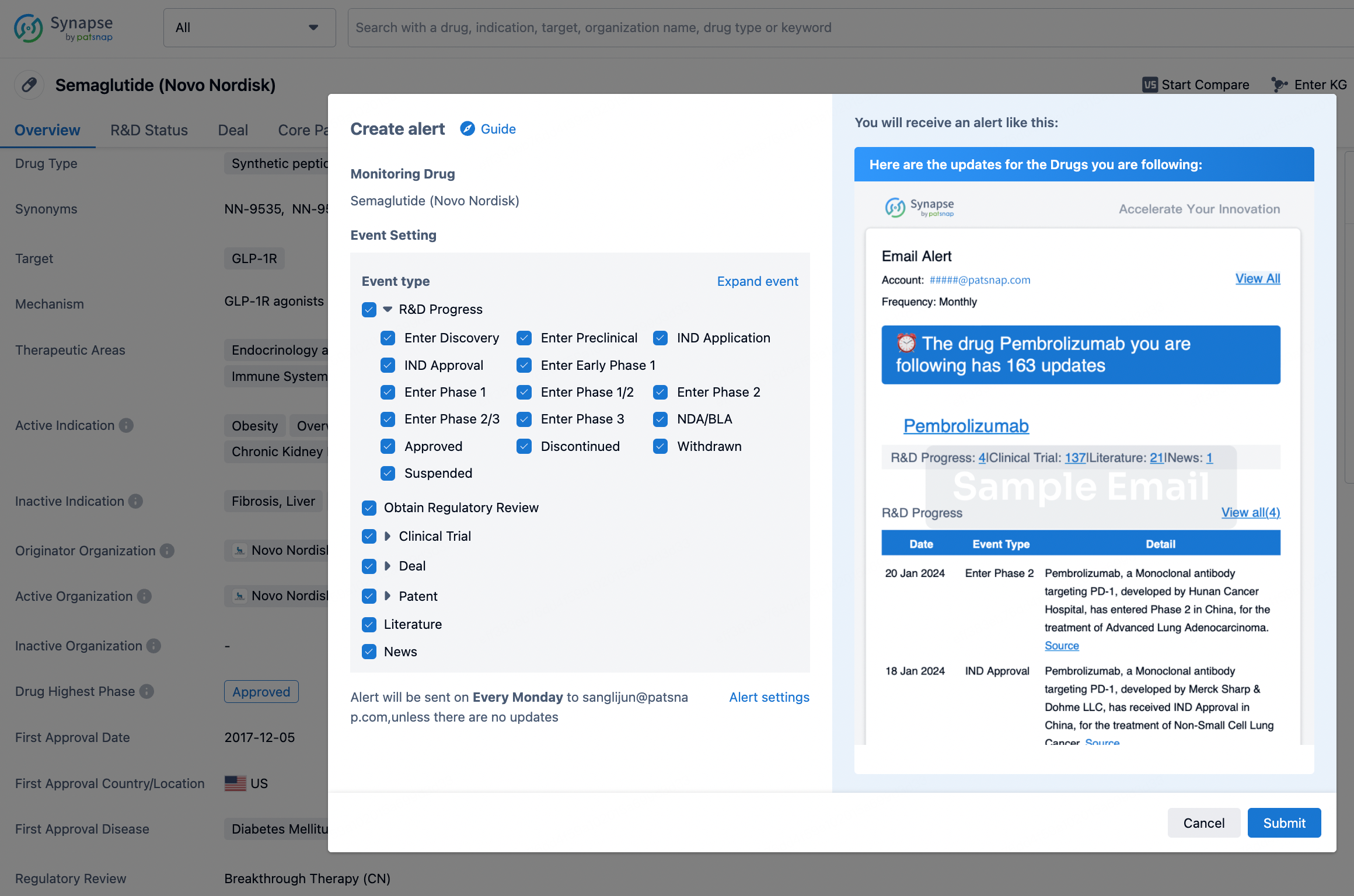
In the Synapse database, you can keep abreast of the latest research and development advances of all drugs anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!