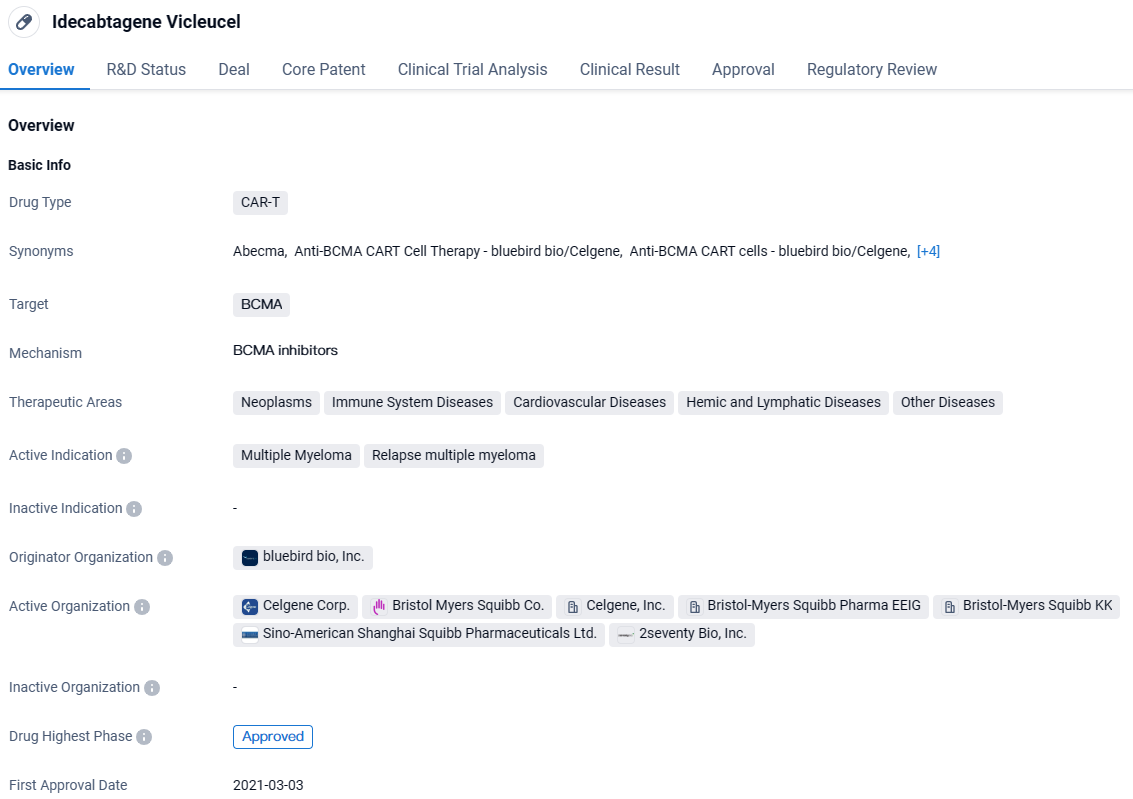
FDA Approves Abecma for Hard-to-Treat Multiple Myeloma Post-Two Therapies
Bristol Myers Squibb together with 2seventy bio, Inc. shared the news that the U.S. Food and Drug Administration (FDA) has given the green light for the use of Abecma® (idecabtagene vicleucel; ide-cel) as a therapeutic option for adults suffering from multiple myeloma that has either relapsed or proven resistant following at least two prior therapy attempts, which must have included treatment with an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody. This approval is rooted in the clinical findings of the KarMMa-3 study.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The authorization of Abecma has been broadened to include its use earlier in the treatment process for individuals who have experienced a relapse or shown resistance following treatment with the three primary classes, post two initial treatment attempts.
"Bryan Campbell, who is the Senior Vice President and the Commercial Head of Cell Therapy at Bristol Myers Squibb, expressed his optimism by stating, ""With a tripled improvement in progression-free survival compared to traditional treatments in cases of relapsed or resistant multiple myeloma, Abecma is set to change the course of therapy for patients much sooner in their treatment timeline.""
Chip Baird, the CEO of 2seventy bio, shared his enthusiasm with the broader accessibility of Abecma for American patients, acknowledging this as a pivotal event for not only the patients and Abecma itself but also for the advances pursued by 2seventy bio in the quest to enhance therapy alternatives and patient outcomes in multiple myeloma.
"The KarMMa-3 trial's findings have been outstanding, particularly when contrasted with the historic results achieved with conventional treatments for these patients dealing with relapsed or resistant diseases," noted Dr. Al-Ola A. Abdallah from the University of Kansas. He holds positions as a Clinical Associate Professor, Clinical Director of Hematologic Malignancies and Cellular Therapeutics, and leads the U.S. Myeloma Innovations Research Collaborative.
Bristol Myers Squibb backs this authorization and its impending extensions by continually investing to boost production capabilities and maintaining an impressively high 94% manufacturing success rate for Abecma in the marketplace.
Notably, Abecma has secured recent approval in Japan, Switzerland, and the European Union for adult patients who have experienced relapse and/or resistance to multiple myeloma after two initial lines of therapy. This positions Abecma distinctly as the singular CAR T cell therapy internationally available for earlier treatment stages in patients harshly affected by triple-class exposed relapsed and/or refractory multiple myeloma.
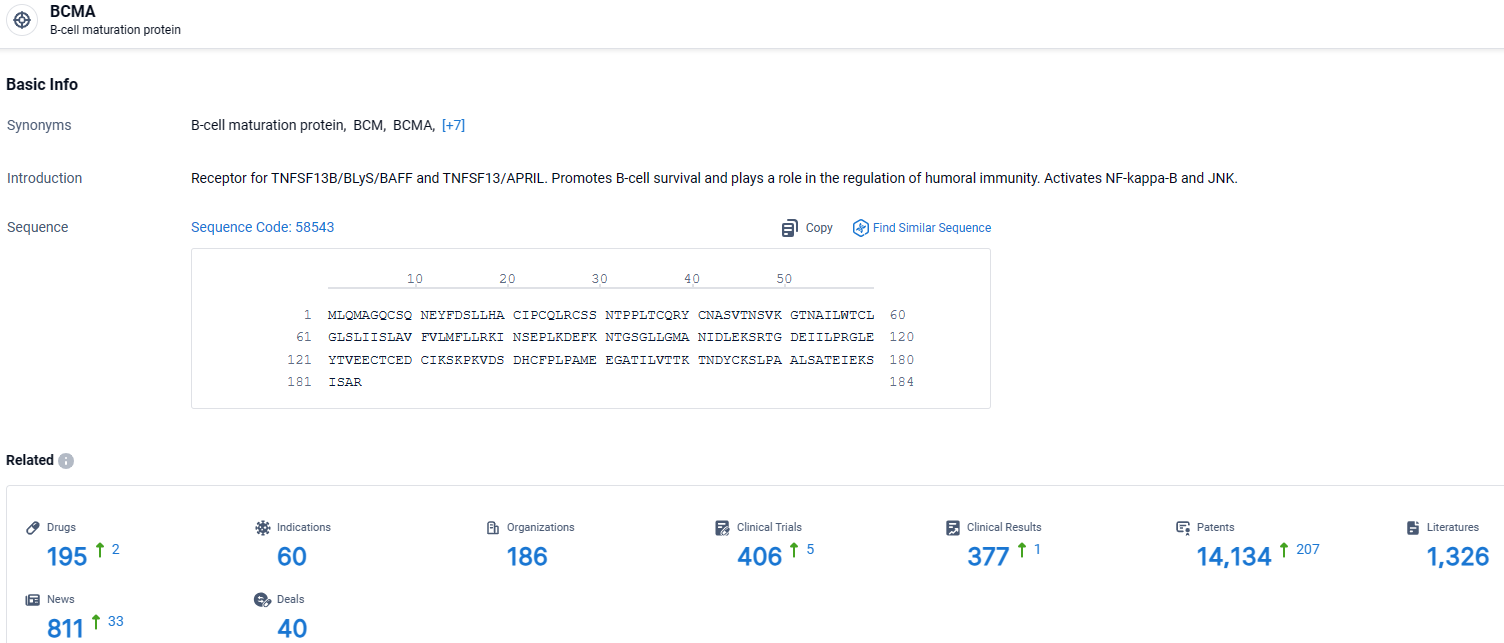
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 7, 2024, there are 195 investigational drugs for the BCMA target, including 60 indications, 186 R&D institutions involved, with related clinical trials reaching 406, and as many as 14134 patents.
Idecabtagene Vicleucel targets BCMA and has been approved for the treatment of multiple myeloma and relapsed multiple myeloma. The drug has shown potential in various therapeutic areas and has received regulatory designations indicating its importance and urgency. Its first approval in the United States in March 2021 marks a significant milestone in the pharmaceutical industry.