Werewolf Therapeutics Unveils Promising Results of WTX-518 and WTX-712 in Cancer Treatment Trial
Werewolf Therapeutics, Inc., a cutting-edge biopharma corporation at the forefront of creating conditionally-activated therapeutic agents that are designed to boost the body's immune response to combat cancer, is showcasing early-stage research findings on their developmental compounds, WTX-518 and WTX-712. These findings will be displayed on poster presentations during the American Association for Cancer Research's (AACR) Annual Meeting, which is scheduled to occur from April 5th to 10th in San Diego, California.
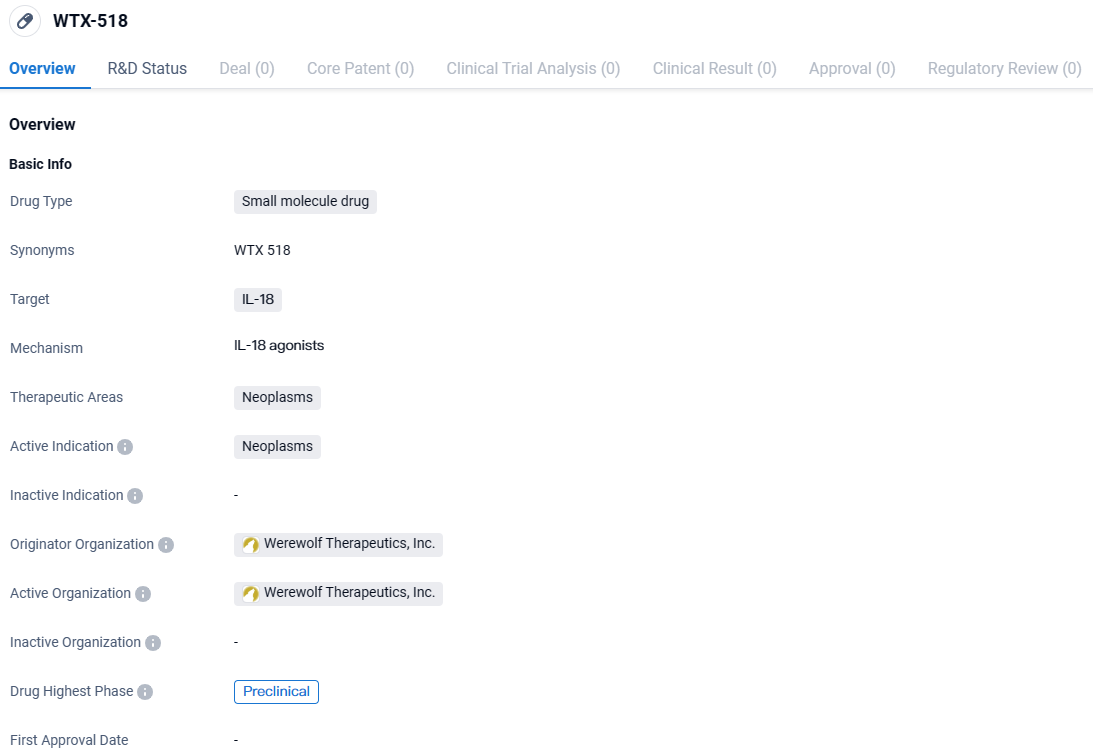
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
WTX-518 has been developed as a highly tumor-selective pro-drug, showing remarkable activation properties within tumor environments, maintaining effectiveness even when facing opposition from IL-18BP, and promoting a strong immune response. This addresses major challenges previously encountered with utilizing such a promising cytokine in the realm of oncology treatment. Meanwhile, WTX-712 introduces a novel approach to immune therapy by specifically targeting and activating tumor-centric T cells. Its mechanism allows for the controlled deployment of unmodified IL-21 within the tumor microenvironment (TME), yielding a wider therapeutic margin. "These findings represent our innovative approach to developing new cytokine-based modalities to energize the immune system in the battle against cancer," stated Daniel J. Hicklin, Ph.D., the head executive at Werewolf.
Research detailing the capabilities of WTX-518 is captured in a presentation titled, “Unveiling of WTX-518, a Tumor Microenvironment-Activated IL-18 Pro-Drug Leading to Tumor Regression in Murine Models”. WTX-518 showcases controllable activation, effectively bypassing the inhibitory effects of IL-18BP and selectively transporting an active form of IL-18 that can resist binding proteins into the TME.
The benefits of WTX-712 are encapsulated in a presentation named, “WTX-712, a Selective IL-21 INDUKINE™ Agent, Promotes Potent Anti-Tumor Responses via a Distinctive Mode of Action”. Essential insights from this include the fact that WTX-712 is inducible, successfully drives tumor shrinkage in the MC38 mouse model, and reveals that IL-21 HLE outperforms IL-2 HLE therapy in certain mouse models resistant to standard anti-PD-1/PD-L1 treatments, attributed in part to the engagement of type-I IFN signaling pathways.
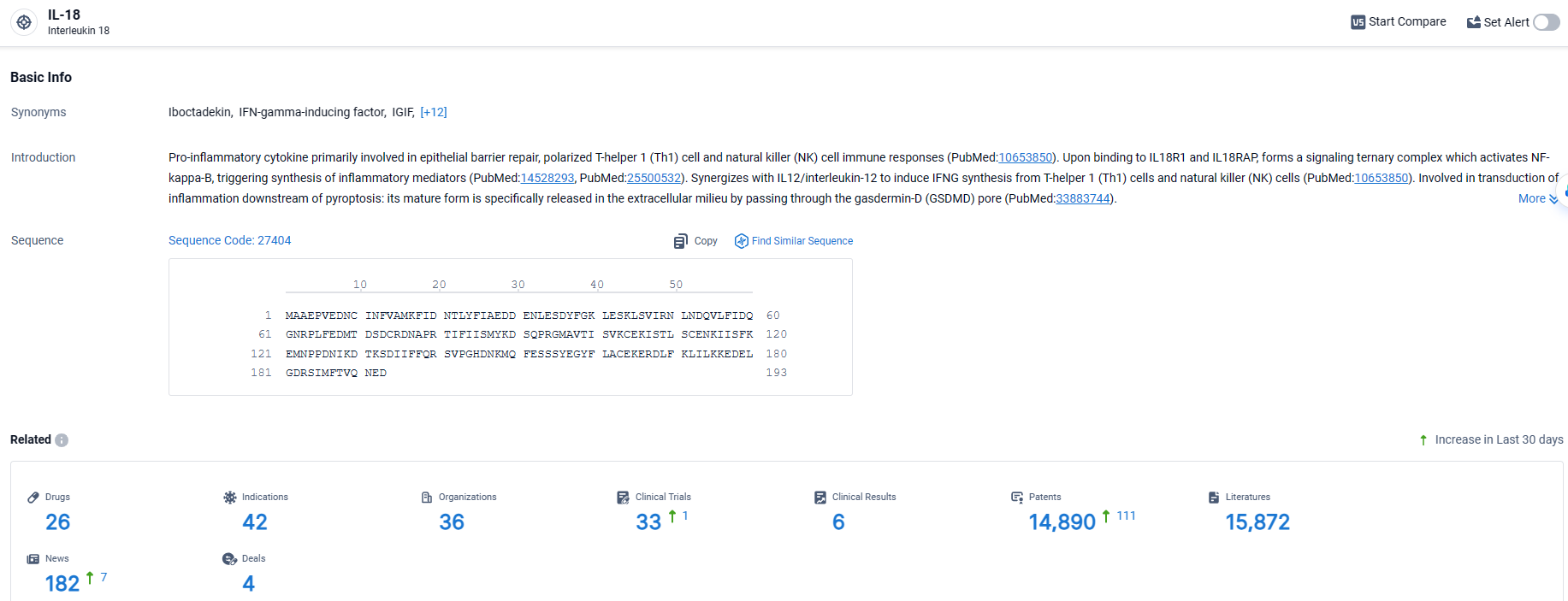
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 7, 2024, there are 26 investigational drugs for the IL-18 target, including 42 indications, 36 R&D institutions involved, with related clinical trials reaching 33, and as many as 14890 patents.
Werewolf Therapeutics, Inc. is the originator organization behind WTX-518. As the developer of the drug, Werewolf Therapeutics is responsible for conducting the necessary research and development activities to bring WTX-518 to market. The company may collaborate with other organizations, such as academic institutions or pharmaceutical companies, to advance the drug's development.