Immunome to Showcase Early Research on Its Primary Radiotherapy Agent, IM-3050, at the AACR Meeting in 2024
Immunome, Inc., a pioneering enterprise in the biotech industry dedicated to advancing novel and superior precision therapies for oncology, has stated its intent to unveil early-stage findings relative to IM-3050, its foremost therapeutic agent incorporating lutetium-177 as a radioligand, designed to hone in on the fibroblast activation protein. Dr. Jack Higgins, the esteemed Chief Scientific Officer of Immunome, is slated to showcase these research insights through a poster session at the 2024 American Association for Cancer Research Annual Meeting, scheduled from April 5th to April 10th in the city of San Diego, California.
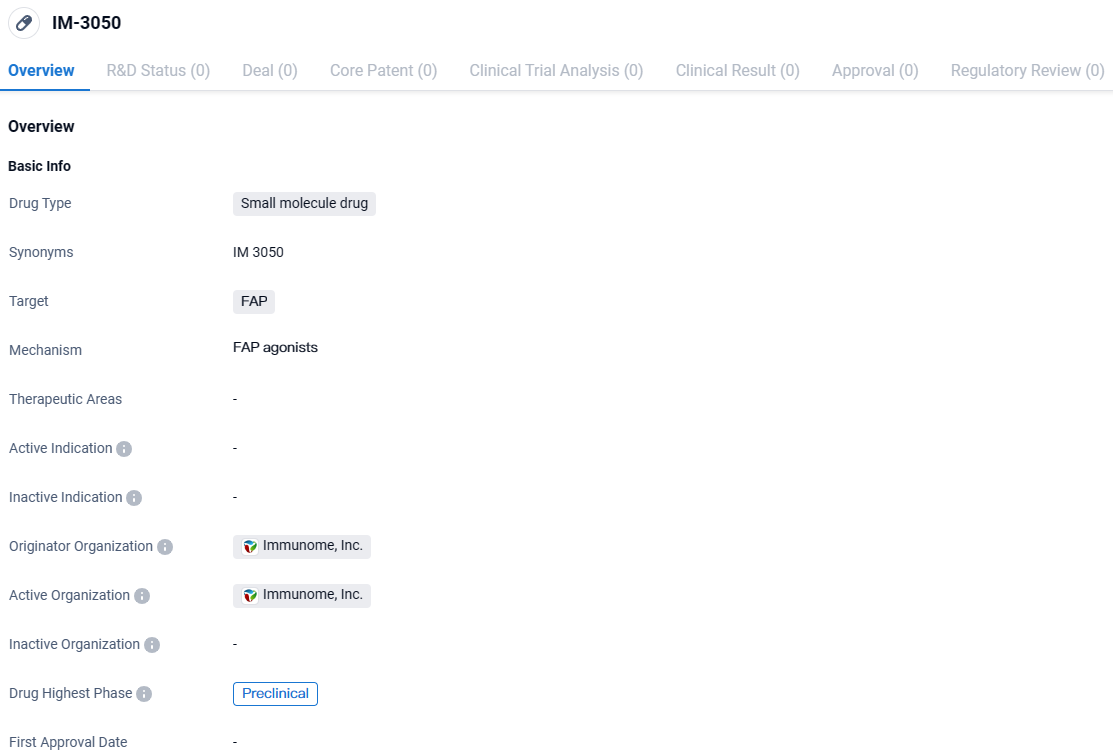
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Dr. Higgins remarked, "FAP represents a compelling objective for oncologic treatments; however, initial FAP-RLTs have delivered only limited patient benefits in trials, highlighting the necessity for advanced compounds with superior therapeutic characteristics." Moreover, Dr. Higgins added, "IM-3050 could emerge as a leader in its category, and we anticipate the moment when we can present the preclinical findings at the AACR conference."
Immunome is planning to file a request for an Investigational New Drug with the FDA for IM-3050 by the start of the second quarter of 2025.
Presently, research on IM-3050 is at the preclinical stage, signifying that it is undergoing first-step developmental processes. During the preclinical phase, the compound undergoes rigorous investigations in laboratories and studies on animal subjects to determine its safety profile, therapeutic effectiveness, and possible adverse reactions. These initial evaluations are critical to ascertain if the compound is viable for progression to human clinical trials.
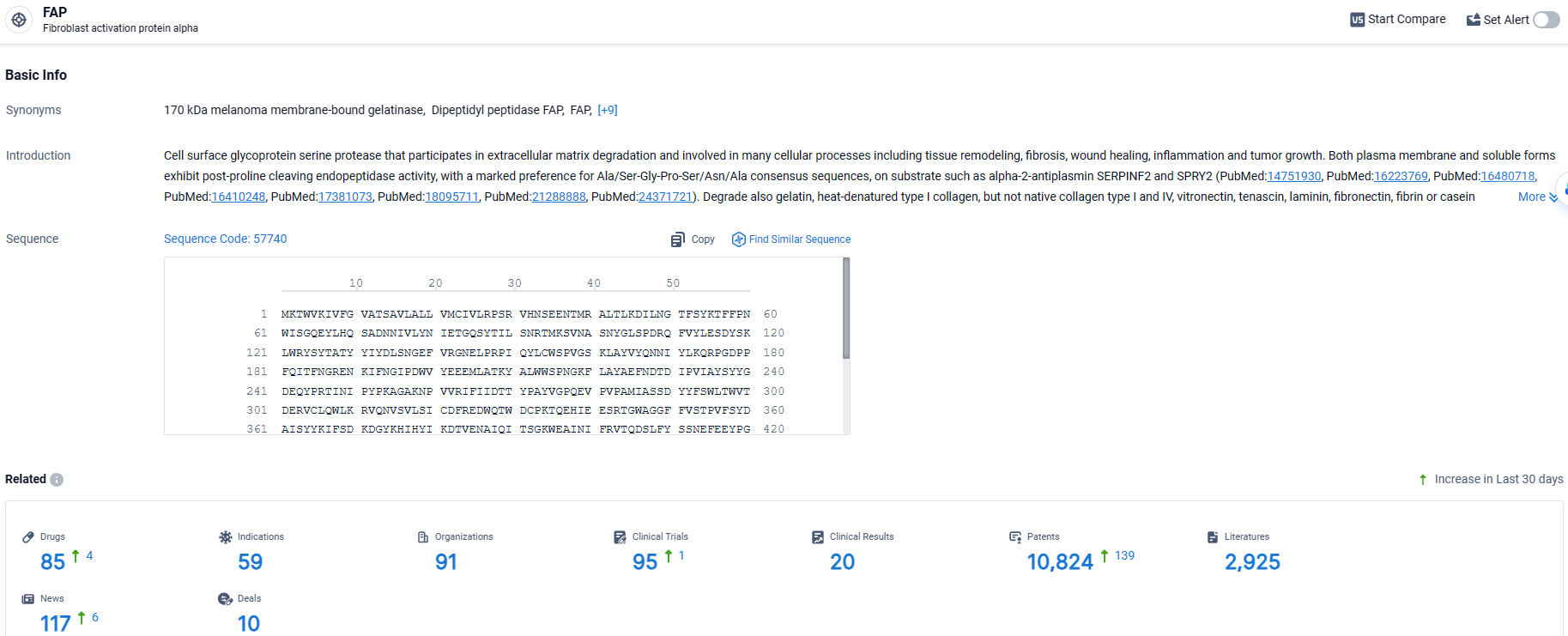
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 7, 2024, there are 85 investigational drugs for the FAP target, including 59 indications, 91 R&D institutions involved, with related clinical trials reaching 95, and as many as 10824 patents.
IM-3050 is a small molecule drug developed by Immunome, Inc. that targets FAP, a protein overexpressed in certain types of cancers. It is currently in the preclinical phase, undergoing laboratory testing to evaluate its safety and efficacy. Further research and development will be necessary to determine its potential as a therapeutic option for cancer treatment.