The small-molecule CXCR4 antagonist Mavorixafor is used for the treatment of WHIM syndrome and chronic neutropenia
Chemokines constitute a large family, primarily composed of small secreted cytokine proteins, that coordinate the migration of white blood cells by interacting with members of the GPCR (G protein-coupled receptor) family on the cell membrane. Chemokines mediate normal host defense and tissue repair but can also support pathological immune responses, including chronic inflammation, autoimmunity, and cancer. The chemokine system is also a primary target for pathogens, such as the Human Immunodeficiency Virus (HIV) and Plasmodium vivax, to evade or exploit the immune system. An increasing number of studies recognize chemokines' additional immune and non-immune functions. Non-immune functions may be advantageous for embryonic development but also detrimental in cancer.
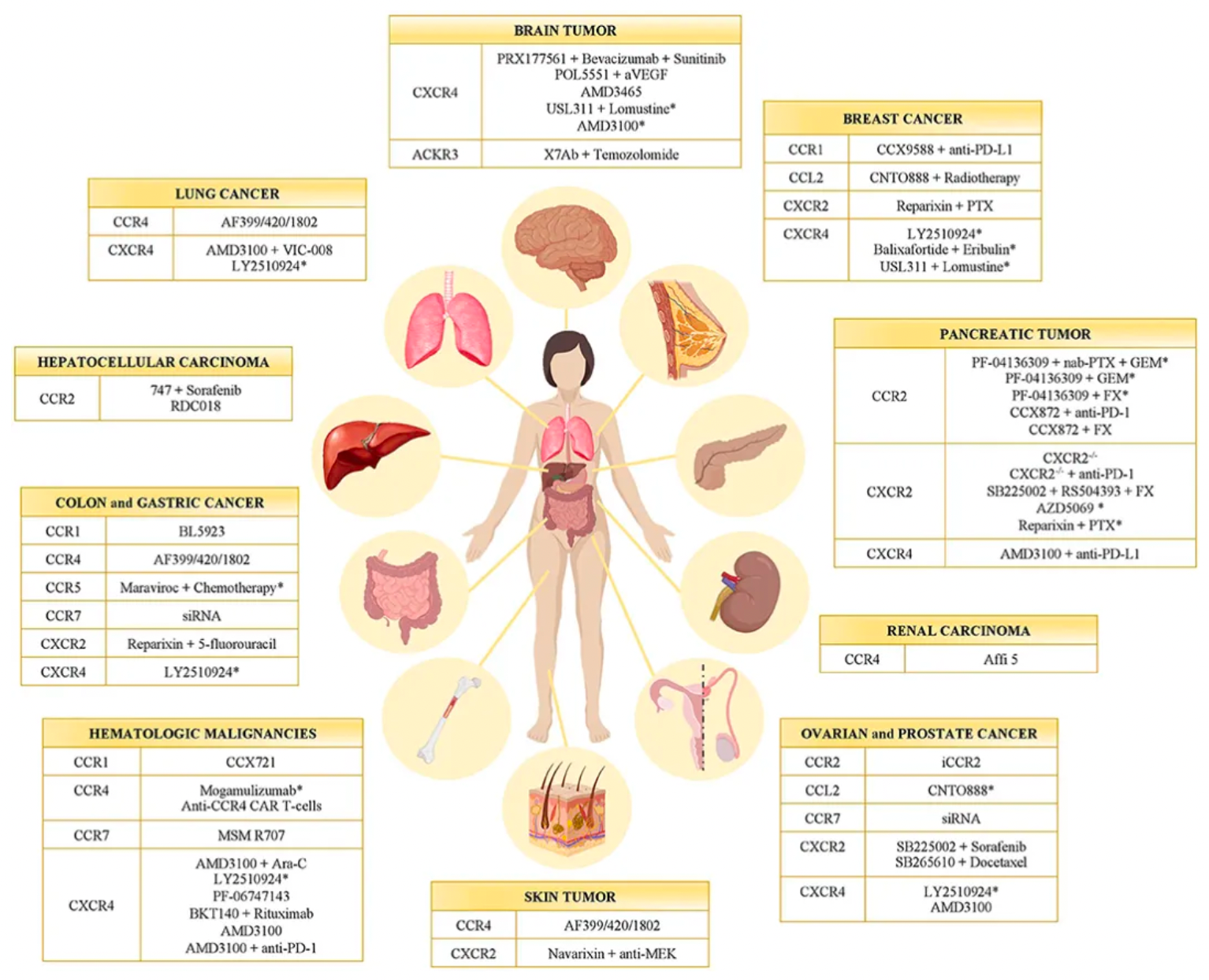
CXCR4 (C-X-C chemokine receptor type 4) is a chemokine receptor belonging to the G protein-coupled receptor family. It plays a pivotal role in regulating the immune system, inflammatory responses, and hematopoietic processes. CXCR4 primarily binds its endogenous ligand CXCL12, participating in a variety of physiological and pathological processes. Clinically, the CXCR4 receptor has become a hot topic of research, as it plays important roles in a multitude of diseases, including cancer metastasis, hematopoietic stem cell transplantation, cardiovascular diseases, and immune disorders.
WHIM syndrome (warts, hypogammaglobulinemia, infections, myelokathexis) is a rare genetic immune disorder characterized by aberrant over-signaling of the CXCR4/CXCL12 pathway, resulting in impaired mobilization and migration of leukocytes in the bone marrow. The acronym of the syndrome reflects its primary features, including warts (warts), hypogammaglobulinemia (hypogammaglobulinemia), recurrent infections (infections), and bone marrow retention (myelokathexis).
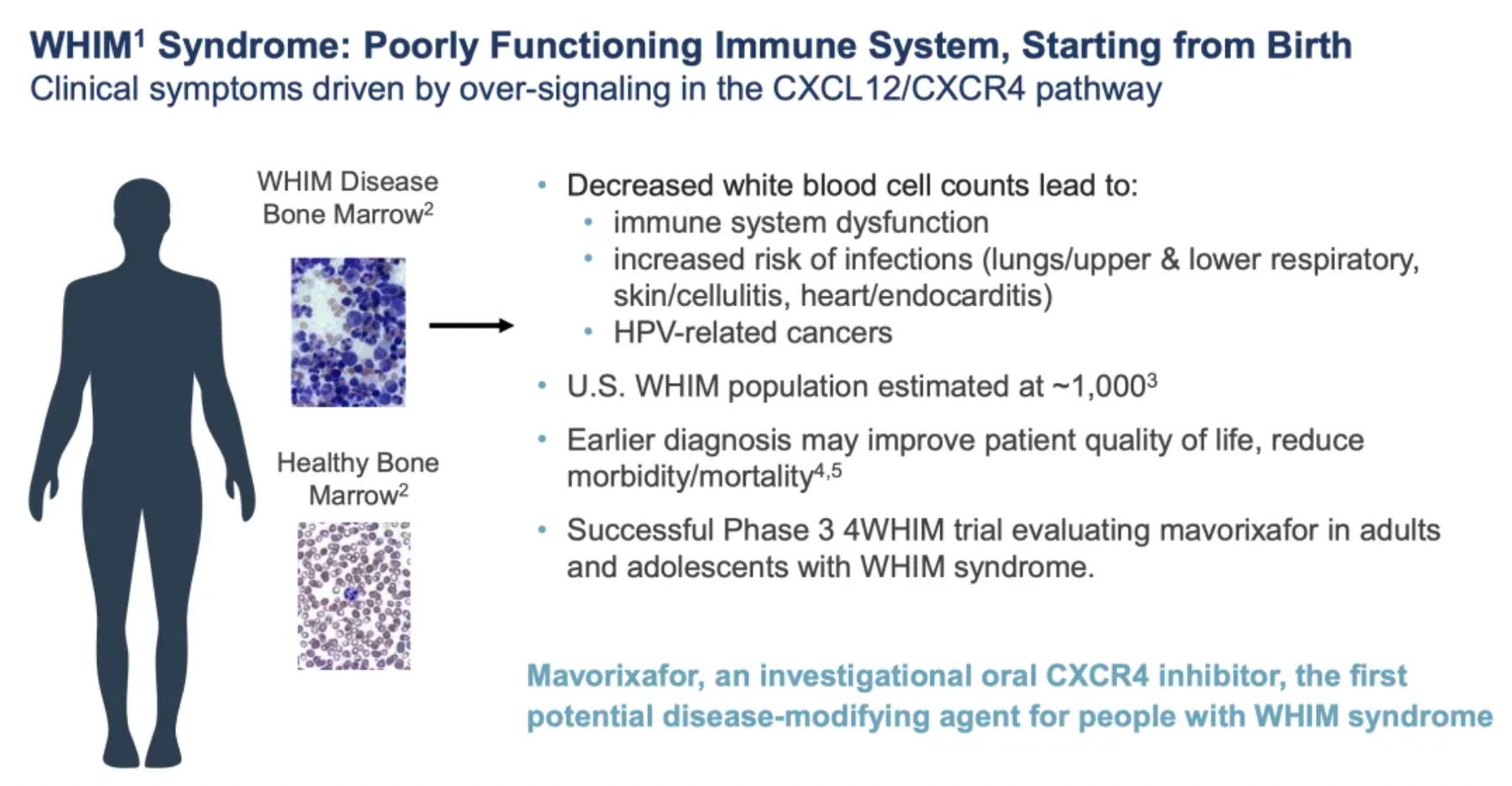
Patients with WHIM syndrome have very low levels of neutrophils (neutropenia) and lymphocytes (lymphopenia) in their blood, making them prone to recurrent infections, at a higher risk for pulmonary diseases, and complications from persistent human papillomavirus (HPV) infections, such as refractory condyloma acuminatum. Additionally, these individuals may experience limited antibody production due to low gammaglobulin levels and have an increased risk of developing certain types of cancer.
In December 2023, X4 Pharmaceuticals issued an announcement stating that its ongoing Phase 2 clinical trial had achieved some initial data. The trial was evaluating the safety and efficacy of once-daily oral mavorixafor in combination with or without the concurrent use of injectable Granulocyte Colony-Stimulating Factor (G-CSF) in patients with idiopathic, cyclic, or congenital neutropenia.

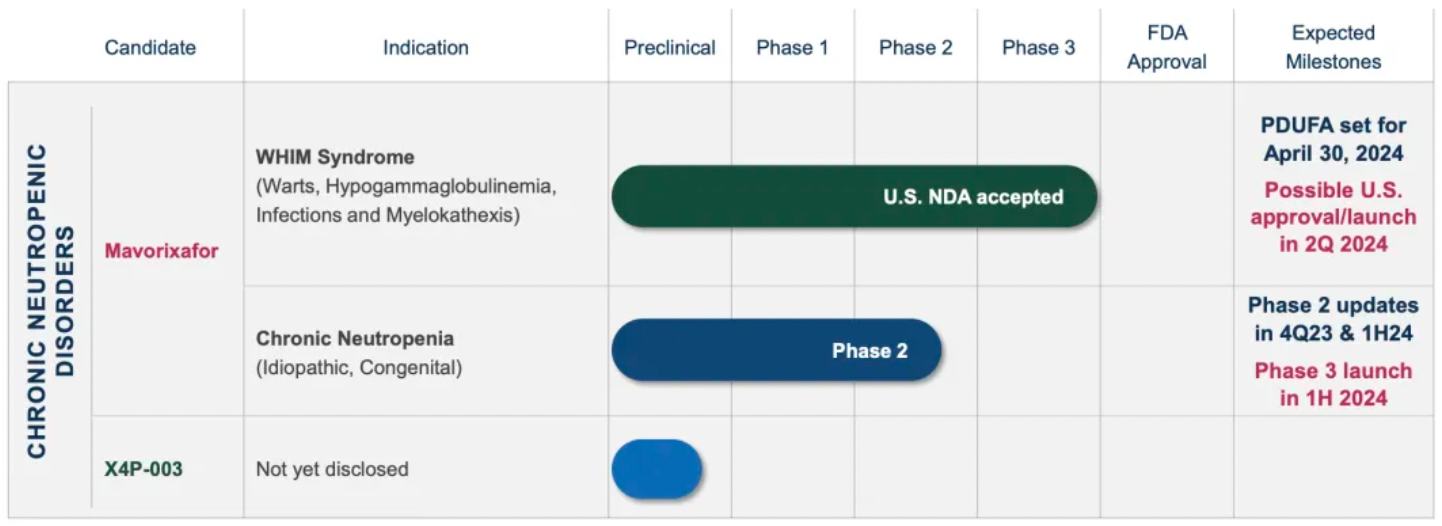
In October 2023, the company announced that the U.S. FDA had accepted for priority review the New Drug Application (NDA) for mavorixafor for the treatment of WHIM syndrome in patients aged 12 and older. The FDA granted priority review status to the mavorixafor NDA, committing to completing the review within six months from the date of acceptance, and set a Prescription Drug User Fee Act (PDUFA) target action date of April 30, 2024.

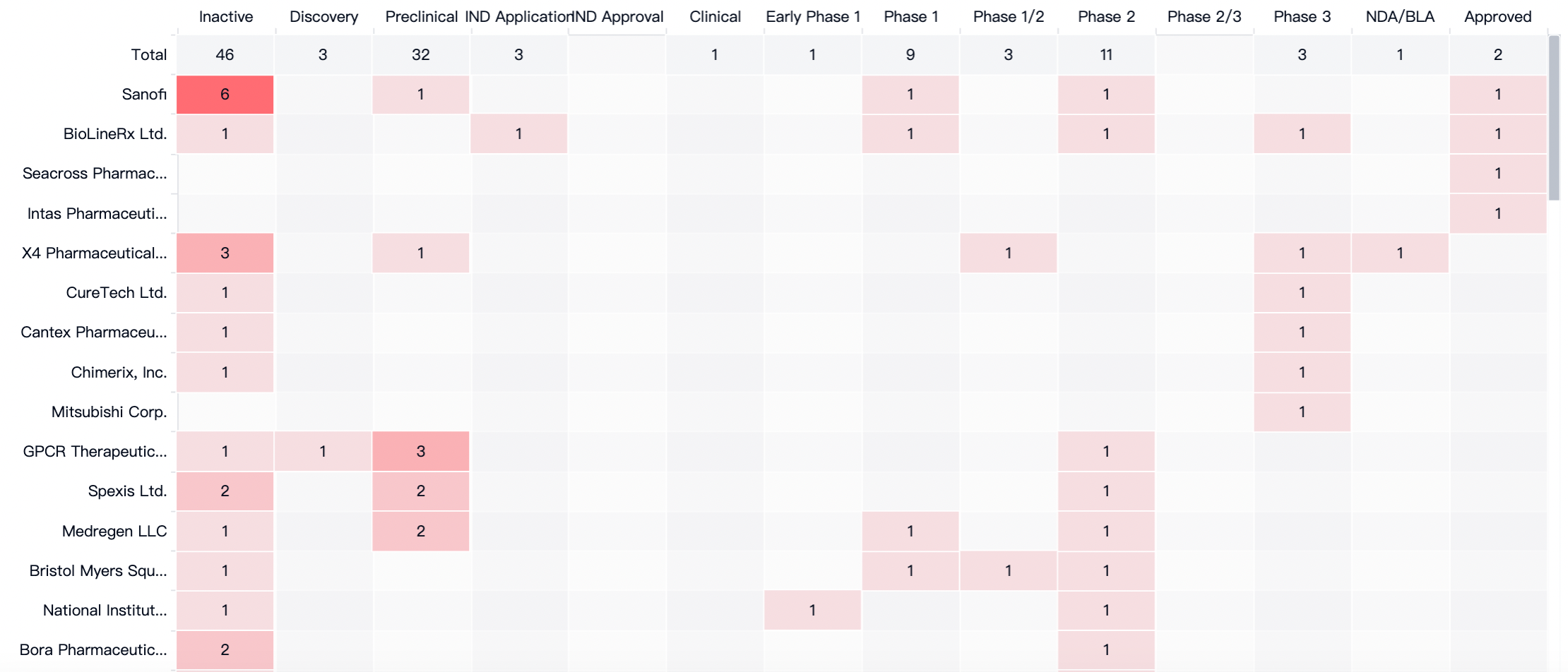
According to statistics from the Synapse database, to date, there are two therapeutic drugs globally targeting CXCR4 that have been approved. These are the small-molecule antagonist Plerixafor by Sanofi and the peptide antagonist Motixafortide.
Mavorixafor is an orally administered small-molecule antagonist targeting the CXCR4 receptor, originally developed by Genzyme Corp., a subsidiary of Sanofi. In 2010, the company published its first research paper on mavorixafor (AMD070, AMD10070) in the Journal of Medicinal Chemistry.

Based on the structure-activity relationship data of existing molecules, researchers, through lead optimization, discovered AMD070: a potent and selective CXCR4 antagonist with an IC(50) value of 13 nM in CXCR4 125I-SDF binding inhibition assays. The compound can inhibit the replication of T-tropic HIV-1 (strain NL4.3) in both MT-4 cells and PBMCs, with IC(50) values of 2 nM and 26 nM, respectively, and remains non-toxic to cells at concentrations exceeding 23 µM. This compound is the first small-molecule, orally bioavailable CXCR4 antagonist developed for the treatment of HIV-1 infection.
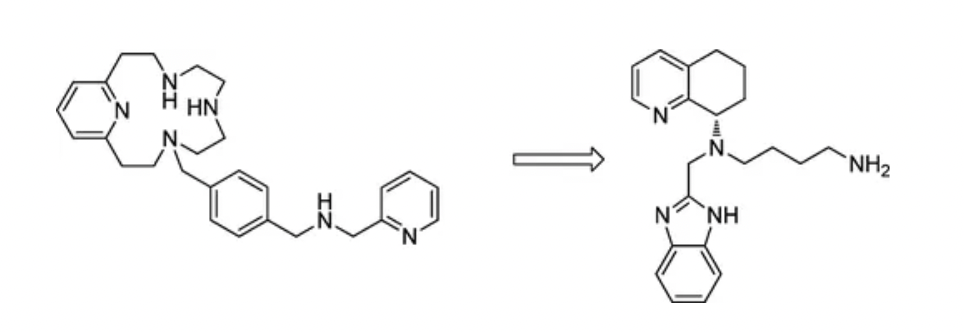
In the subsequent clinical data released, it was demonstrated that AMD10070 has certain antiviral activity against tropical HIV-1, with good tolerability and no serious safety concerns. However, due to histological changes observed in the liver tissue in long-term animal studies, AMD11070 is currently on clinical hold; further preclinical safety evaluations are underway.

In 2017, X4 Pharmaceuticals raised $27 million to advance the CXCR4 inhibitor mavorixafor, which was acquired from Sanofi. According to the latest materials updated by X4 Pharmaceuticals, the company is now conducting trials for the use of mavorixafor in treating WHIM syndrome and chronic neutropenia.
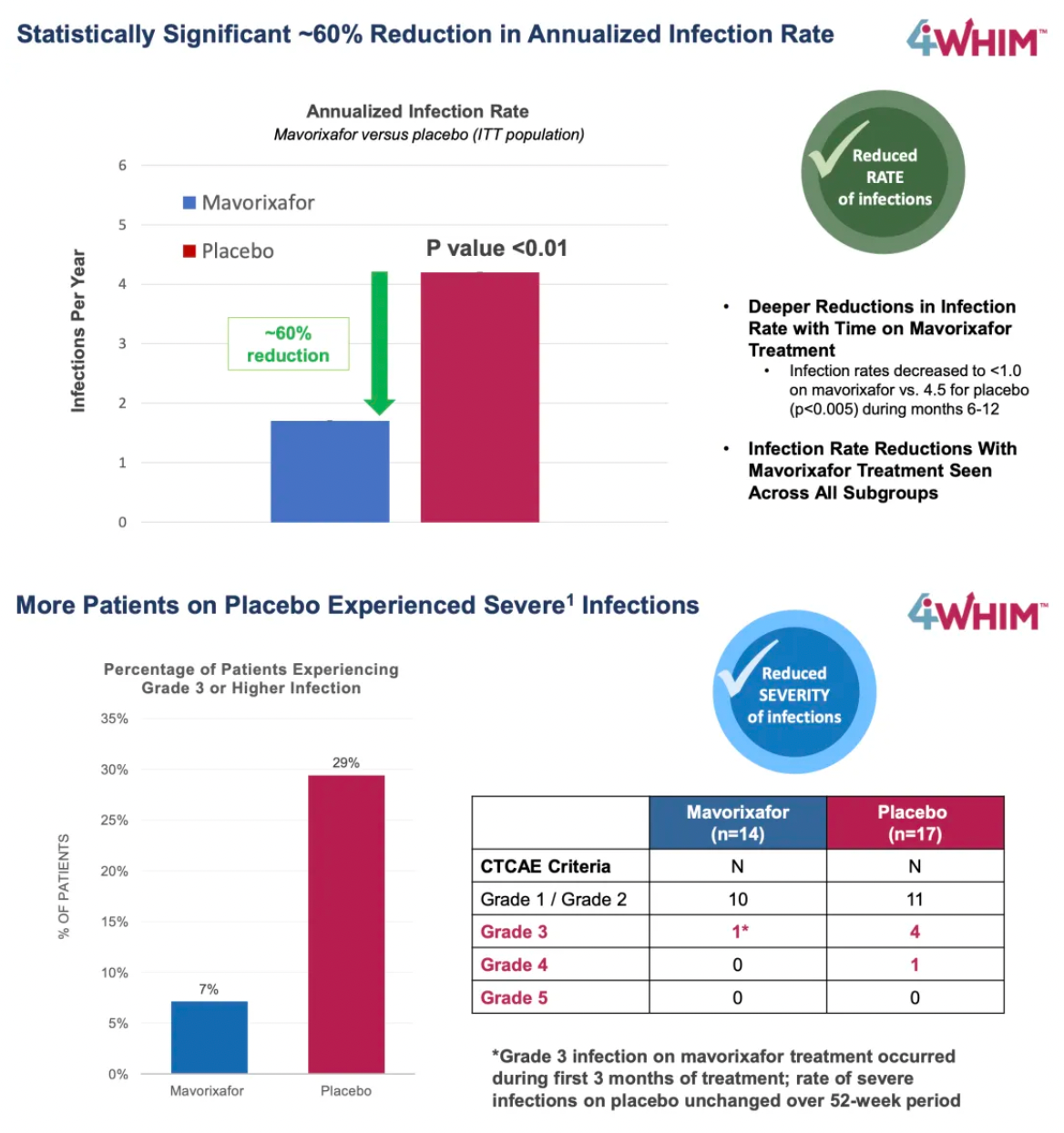
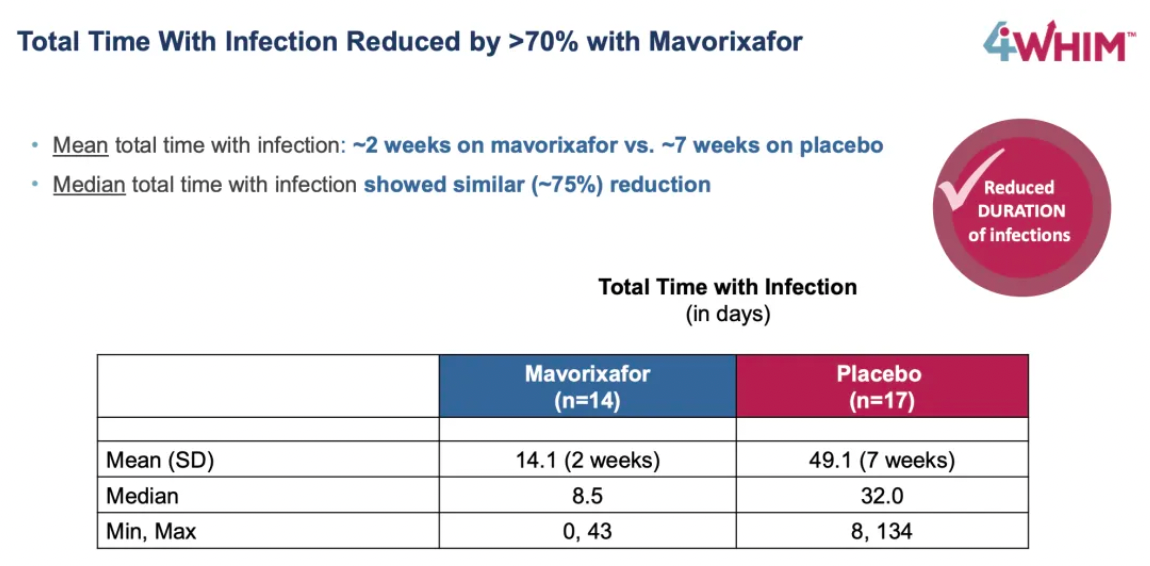
The 4WHIM trial achieved its primary endpoints, namely the duration of absolute neutrophil counts (ANC) exceeding thresholds and certain key clinical efficacy assessments.
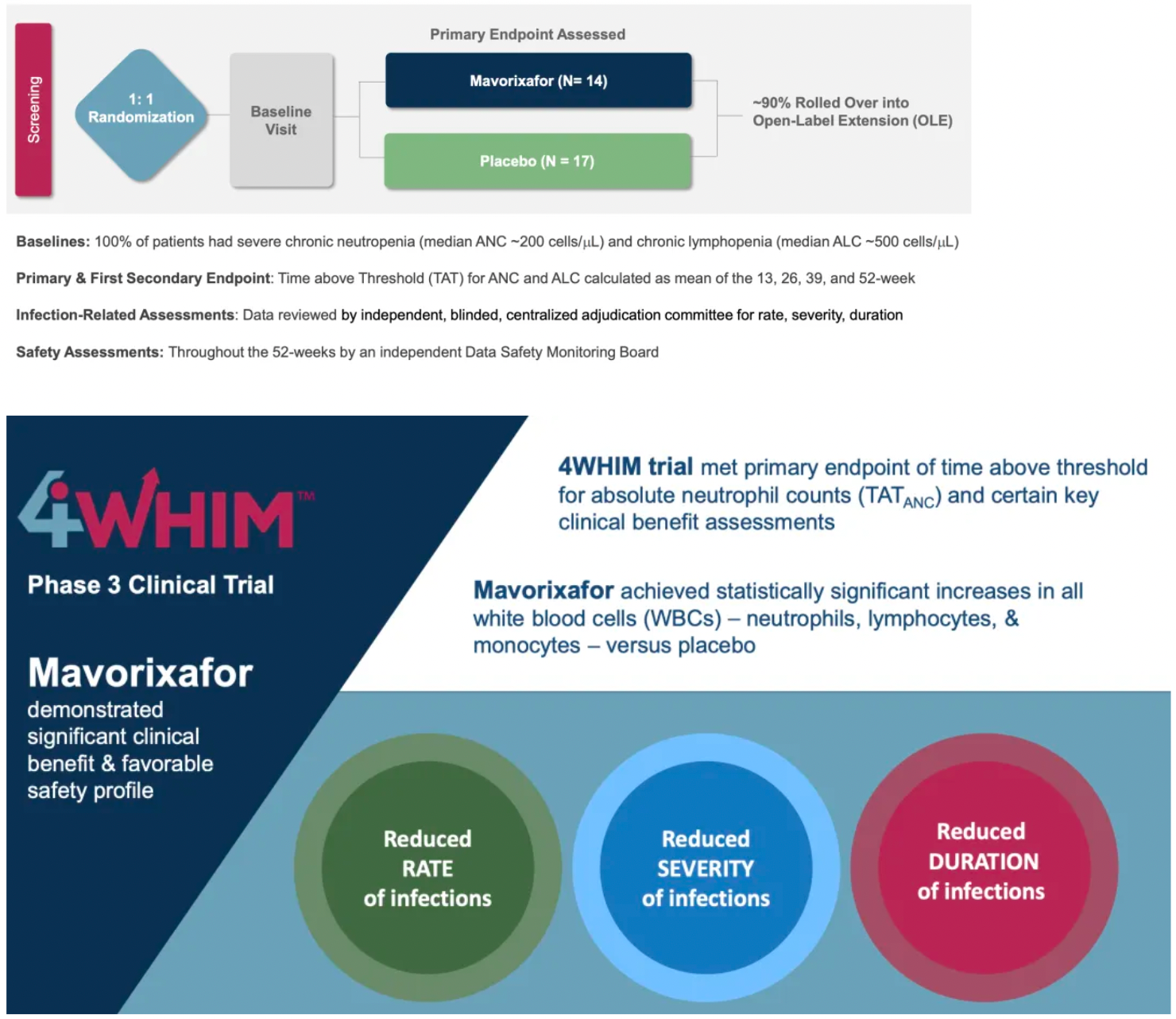
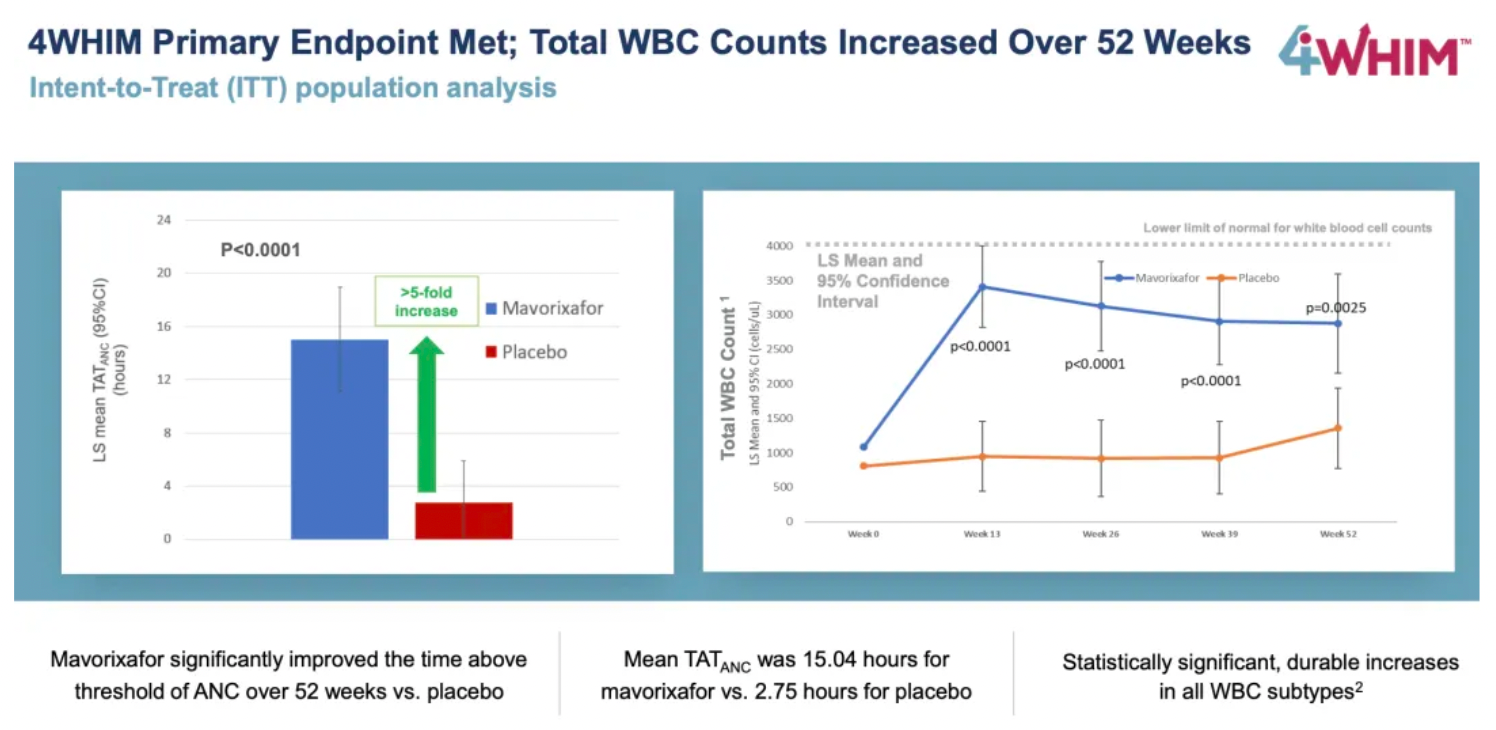
Compared to placebo, mavorixafor significantly reduced the time with ANC above the critical threshold within 52 weeks, with an average TATANC (Time Above Threshold Absolute Neutrophil Count) of 15.04 hours longer than the 2.75 hours in the placebo group.
The longer mavorixafor was used, the greater the reduction in infection rates.