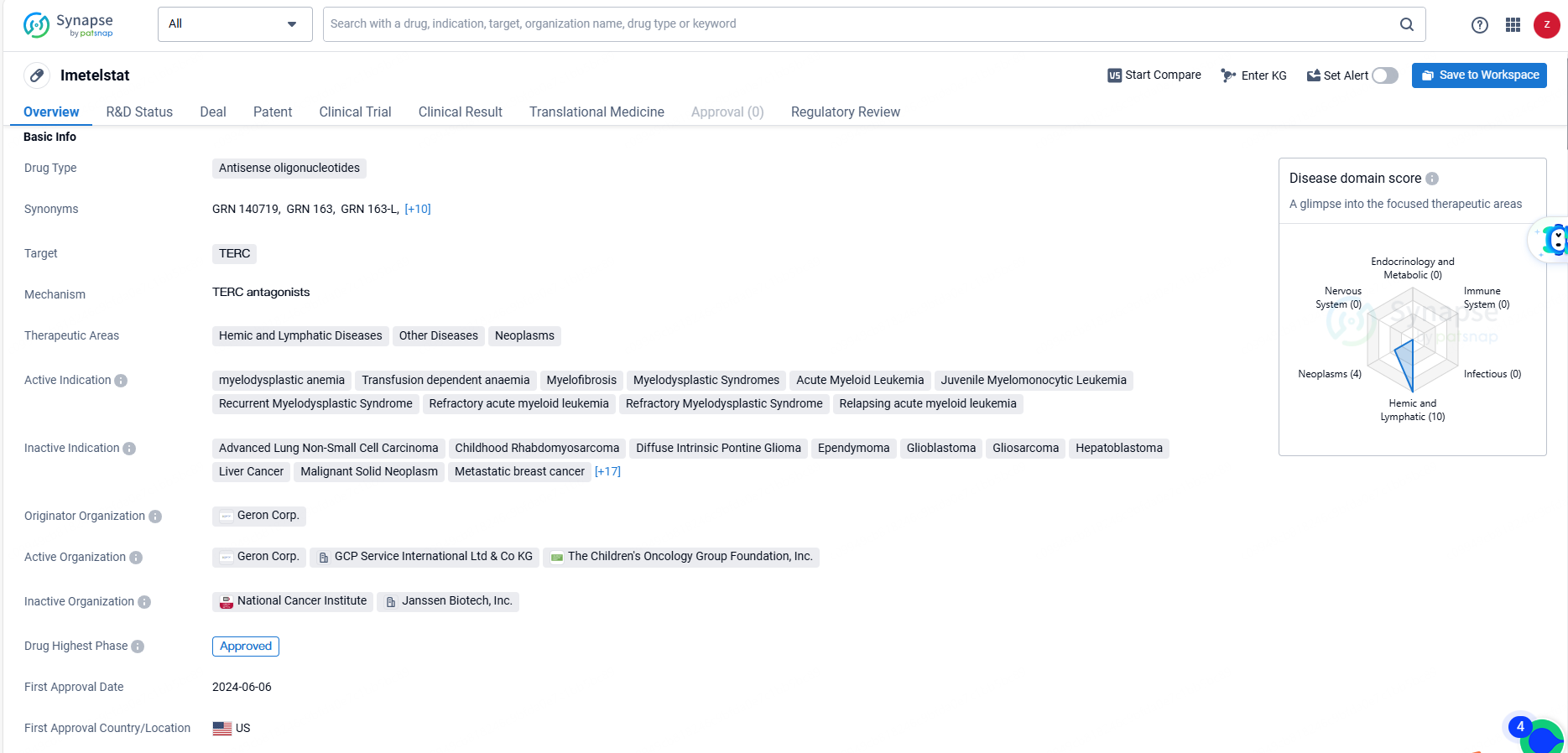
FDA Approves Geron's RYTELO™ for Treating Anemia in Lower-Risk MDS
Geron Corporation a biopharmaceutical company at the commercial stage focused on transforming the lives of those affected by blood cancers, announced today that the U.S. Food and Drug Administration has granted approval for RYTELO™ (imetelstat). This medication is indicated for adult patients suffering from low- to intermediate-1 risk myelodysplastic syndromes with transfusion-dependent anemia, who require four or more units of red blood cells every eight weeks and have not responded to, have lost response to, or are unsuitable for treatment with erythropoiesis-stimulating agents.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
John A. Scarlett, M.D., Geron's Chairman and Chief Executive Officer, stated, "With RYTELO's approval and accessibility, we are optimistic that qualified patients with lower-risk MDS may gain significant clinical advantages. This includes the potential to be free from the need for red blood cell transfusions and the symptoms of anemia for more than 24 weeks."
Dr. Scarlett elaborated, "RYTELO's approval as the inaugural telomerase inhibitor showcases the strength of our scientific innovations and the dedication of our team to progress in blood cancer treatment. As we mark this significant achievement, I want to express my gratitude to the patients, their families, advocates, healthcare professionals, study coordinators, site staff, scientists, and both current and former Geron employees and collaborators who played a crucial role in this milestone and our evolution into a commercial entity."
Lower-risk myelodysplastic syndromes (LR-MDS) are types of blood cancers that often escalate, necessitating more rigorous management of core symptoms such as anemia and consequent fatigue. Patients with symptomatic LR-MDS frequently require regular red blood cell transfusions, a dependency associated with adverse short- and long-term clinical outcomes, potentially diminishing quality of life and lifespan.
There remains a considerable unmet necessity for many LR-MDS patients, especially those with prognostic factors indicating poor outcomes. For those who do not respond to erythropoiesis-stimulating agents (ESA), existing treatment options are limited to specific sub-groups, underscoring the need for therapies offering prolonged and consistent independence from red blood cell transfusions.
The FDA's authorization of RYTELO is founded on the IMerge Phase 3 clinical trial results, which were documented in The Lancet. The IMerge trial achieved its primary and key secondary endpoints, revealing that RYTELO significantly increased rates of red blood cell transfusion independence compared to a placebo, for a minimum of eight consecutive weeks and notably, 24 weeks. The durability of RBC-TI in the RYTELO group was evident, with median durations for 8-week and 24-week responders being approximately one year and 1.5 years, respectively.
RYTELO™ (imetelstat) received FDA approval as an oligonucleotide telomerase inhibitor for adult patients with low-to-intermediate-1 risk myelodysplastic syndromes (LR-MDS) and transfusion-dependent anemia, requiring four or more red blood cell units over eight weeks, who have either not responded to or lost response to, or are unsuitable for erythropoiesis-stimulating agents. It is intended to be administered as a two-hour intravenous infusion every four weeks.
As a pioneering treatment, RYTELO works by inhibiting telomerase enzyme activity. Telomeres are protective structures at the ends of chromosomes that naturally shorten with each cell division. In LR-MDS, abnormal bone marrow cells often produce telomerase, which reconstructs these telomeres, enabling unregulated cell division. Developed and solely owned by Geron, RYTELO is the first and only telomerase inhibitor approved by the U.S. Food and Drug Administration.
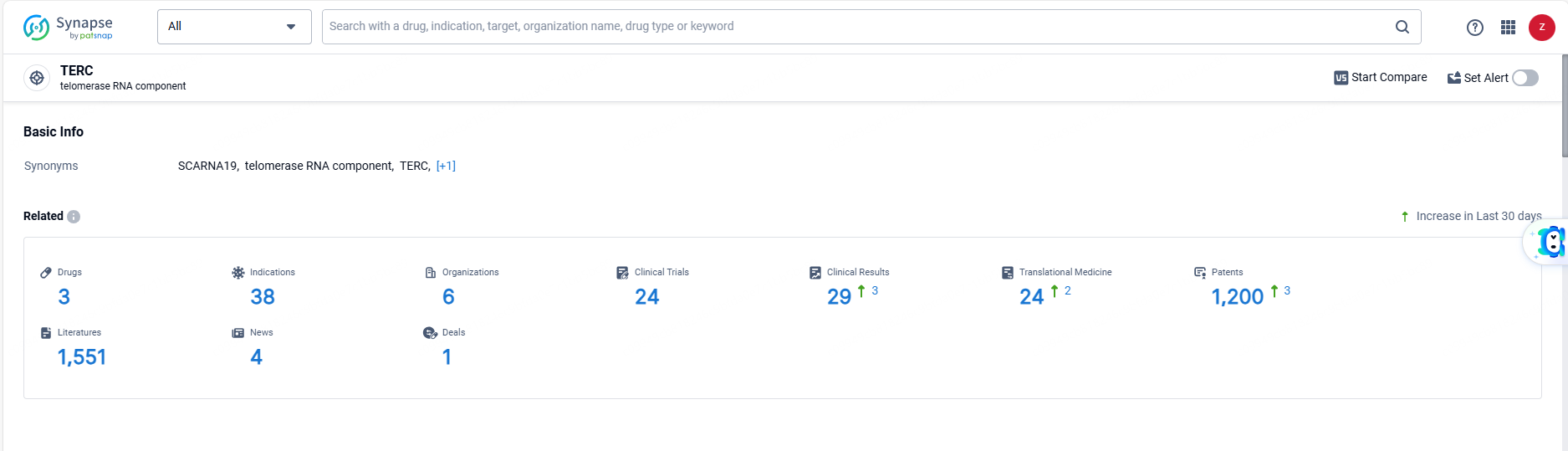
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 13, 2024, there are 3 investigational drugs for the TERC target, including 38 indications, 6 R&D institutions involved, with related clinical trials reaching 24, and as many as 1200 patents.
Imetelstat represents a novel approach to targeting TERC and inhibiting telomerase activity, which is often dysregulated in cancer and hematologic disorders. Its Fast Track and Orphan Drug designations further highlight the potential impact of this drug in addressing unmet medical needs and improving outcomes for patients with rare and challenging conditions.