FDA Approves Biocon's YESINTEK™ Biosimilar to Stelara®
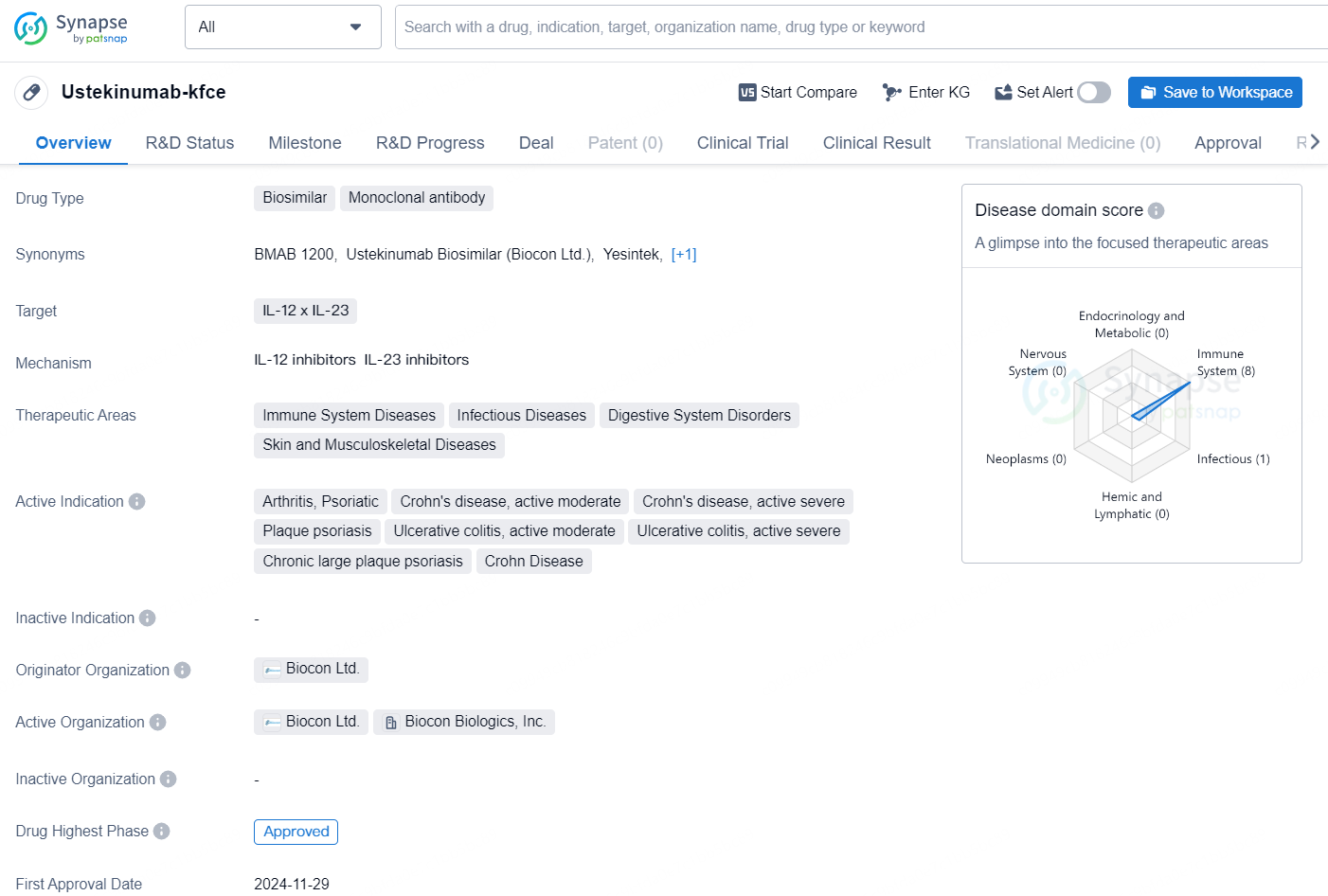
Biocon Biologics Ltd (BBL), a comprehensive global player in the biosimilars market and a subsidiary of Biocon Ltd, has revealed that the U.S. Food and Drug Administration (FDA) has granted approval for YESINTEK™ (Ustekinumab-kfce), which is a biosimilar to the reference medication, Stelara® (Ustekinumab).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
YESINTEK™, a monoclonal antibody, is sanctioned for the management of Crohn’s disease, Ulcerative Colitis, Plaque Psoriasis, and Psoriatic Arthritis.
On February 29, 2024, Biocon Biologics Ltd informed the Stock Exchange that it had reached a settlement and licensing deal with Janssen Biotech Inc., Janssen Sciences Ireland, and Johnson & Johnson (referred to collectively as Janssen) to bring YESINTEK™ to market in the United States by February 22, 2025, contingent upon receiving approval from the U.S. FDA.
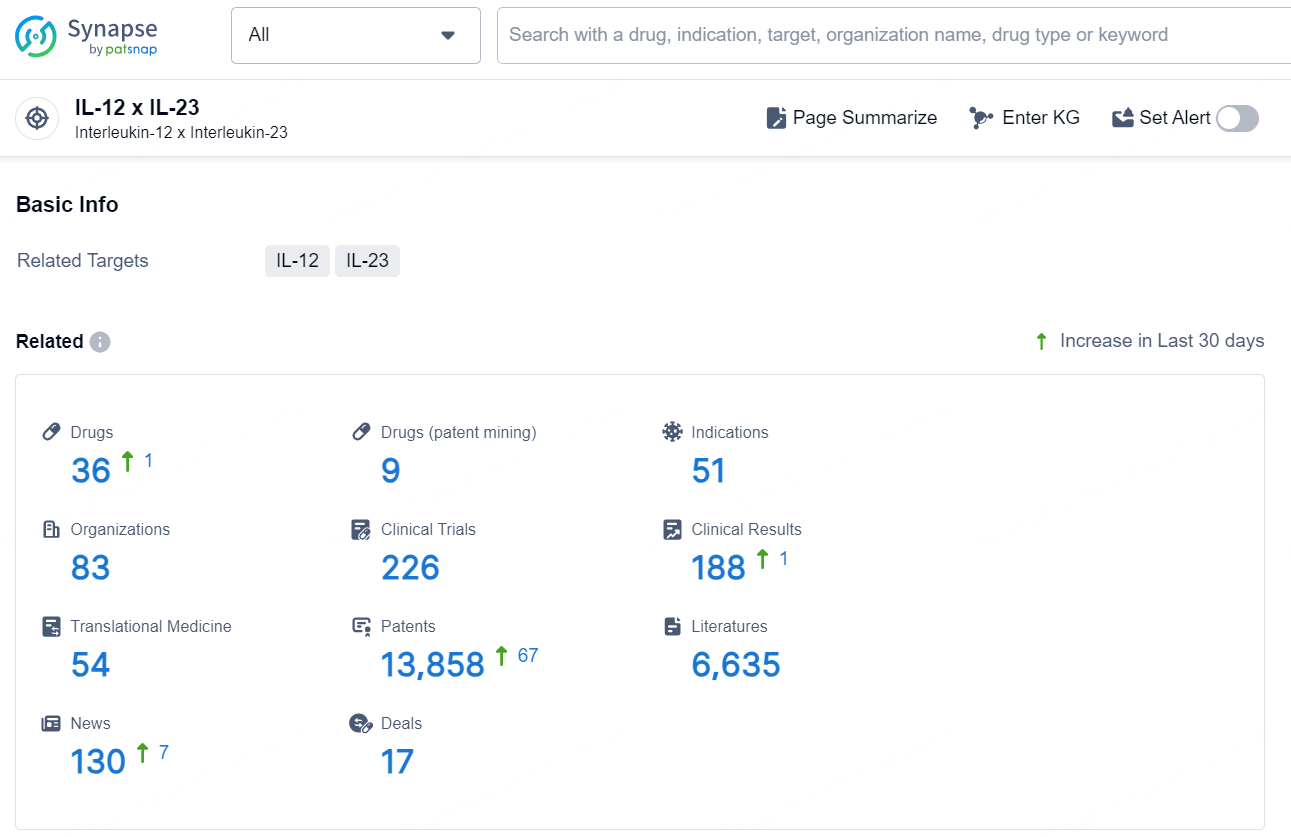
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 6, 2024, there are 36 investigational drugs for the IL-12 and IL-23 target, including 51 indications, 83 R&D institutions involved, with related clinical trials reaching 226, and as many as 13858 patents.
Ustekinumab-kfce is a biosimilar drug classified as a monoclonal antibody, designed to target IL-12 and IL-23. This drug has been developed to address a range of therapeutic areas including immune system diseases, infectious diseases, digestive system disorders, as well as skin and musculoskeletal diseases.