FDA Approves ImmunityBio’s ANKTIVA® for BCG-Resistant Bladder Cancer
ImmunityBio, Inc., a company specializing in immunotherapy, disclosed that ANKTIVA (N-803 or nogapendekin alfa inbakicept-pmln) combined with Bacillus Calmette-Guérin has received approval from the U.S. Food and Drug Administration. This approval is for treating individuals who have non-muscle invasive bladder cancer characterized by carcinoma in situ, who are non-responsive to BCG, with or without the presence of papillary tumors.
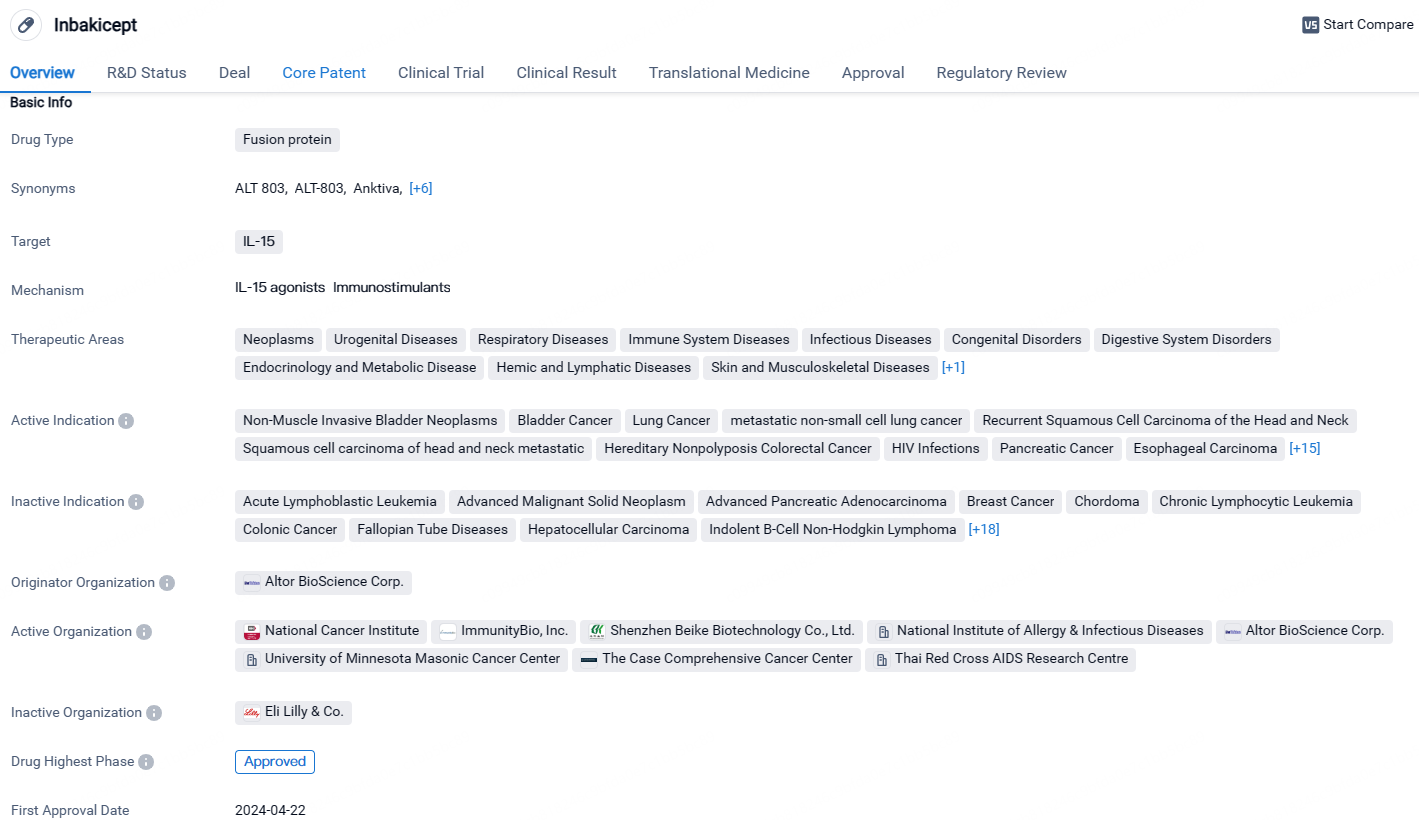
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Patrick Soon-Shiong, M.D., Executive Chairman and Global Chief Scientific and Medical Officer at ImmunityBio, announced the FDA’s endorsement of ANKTIVA, initiating their debut of an advanced immunotherapy that extends beyond traditional checkpoint inhibitors. "ANKTIVA not only stimulates the growth and activation of a patient's native NK cells and CD8+ killer T cells but also promotes the activation of CD4+ T helper cells, thereby augmenting the expansion of memory killer T cells,” he stated.
He further explained, “This unique action mechanism, which resembles the biological activities of dendritic cells, sets the stage for the advancement of immunotherapy that includes more than just T cells. Combining the expansive proliferation of critical cancer-fighting immune cells with the activation of long-lasting memory T cells fosters enduring complete responses. Our strategy to create a cancer therapeutic vaccine effective against multiple types of tumors, regardless of their initial location, is anchored in this ‘triangle offense’ of tumor elimination facilitated by enduring immune memory.”
ANKTIVA, a pioneering IL-15 agonist immunotherapy for NMIBC, received both Breakthrough Therapy Designation and FDA approval based on compelling safety profiles, efficacy data showing complete responses, and the sustainability of these responses. In this single-arm, multicenter study involving 77 evaluable patients, ANKTIVA combined with BCG maintenance therapy was administered for up to 37 months. The monitoring of tumor status was performed using cystoscopy and urine cytology and is set to continue for up to five years from the start of each participant's involvement in the trial.
Roger Buckley from the IBCG commented, “The achievement that ANKTIVA’s treatment surpasses the clinically important criteria set by the IBCG in 2016 for a sustained complete response is gratifying. We anticipate the global roll-out of ANKTIVA which could lessen the frequency of cystectomy for numerous NMIBC patients worldwide.”
Karim Chamie, M.D., Associate Professor of Urology at UCLA and lead investigator of the QUILT 3.032 trial remarked, “The extended period of complete response, exceeding 47 months, revolutionizes the treatment landscape for NMIBC patients and underscores the significant potency of ANKTIVA for those traditionally facing high recurrence rates and substantial decreases in quality of life from invasive surgeries. ANKTIVA’s approval may well set a new therapeutic benchmark for NMIBC management and has the potential to redefine bladder cancer treatment protocols.”
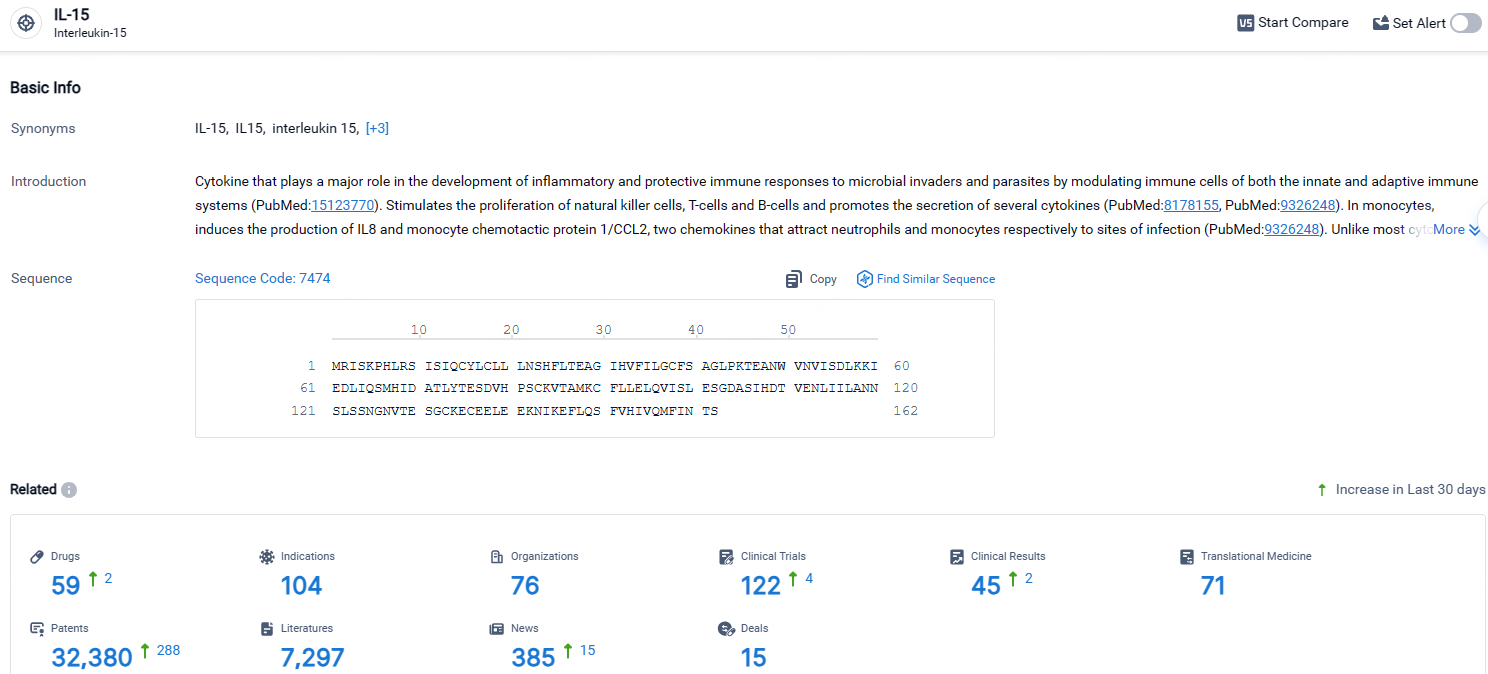
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 24, 2024, there are 59 investigational drugs for the IL-15 targets, including 104 indications, 76 R&D institutions involved, with related clinical trials reaching 122, and as many as 32380 patents.
Inbakicept is a fusion protein drug that targets IL-15 and has shown promising therapeutic potential in a wide range of diseases, particularly various types of cancers. With its global approval and regulatory designations, it is expected to provide a valuable treatment option for patients in need. The ongoing Phase 2 trial in China indicates the potential for future approvals in additional markets.