Neurogene to Present Early Results on NGN-401 Gene Therapy for Rett Syndrome at ASGCT Conference
Neurogene Inc. a clinical-stage enterprise dedicated to developing transformative genetic treatments for individuals and families grappling with rare neurological disorders, disclosed that preliminary data on safety and tolerability from its active Phase 1/2 gene therapy trial targeting Rett syndrome is set to be showcased at the upcoming Annual Meeting of the American Society for Gene and Cell Therapy.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately. 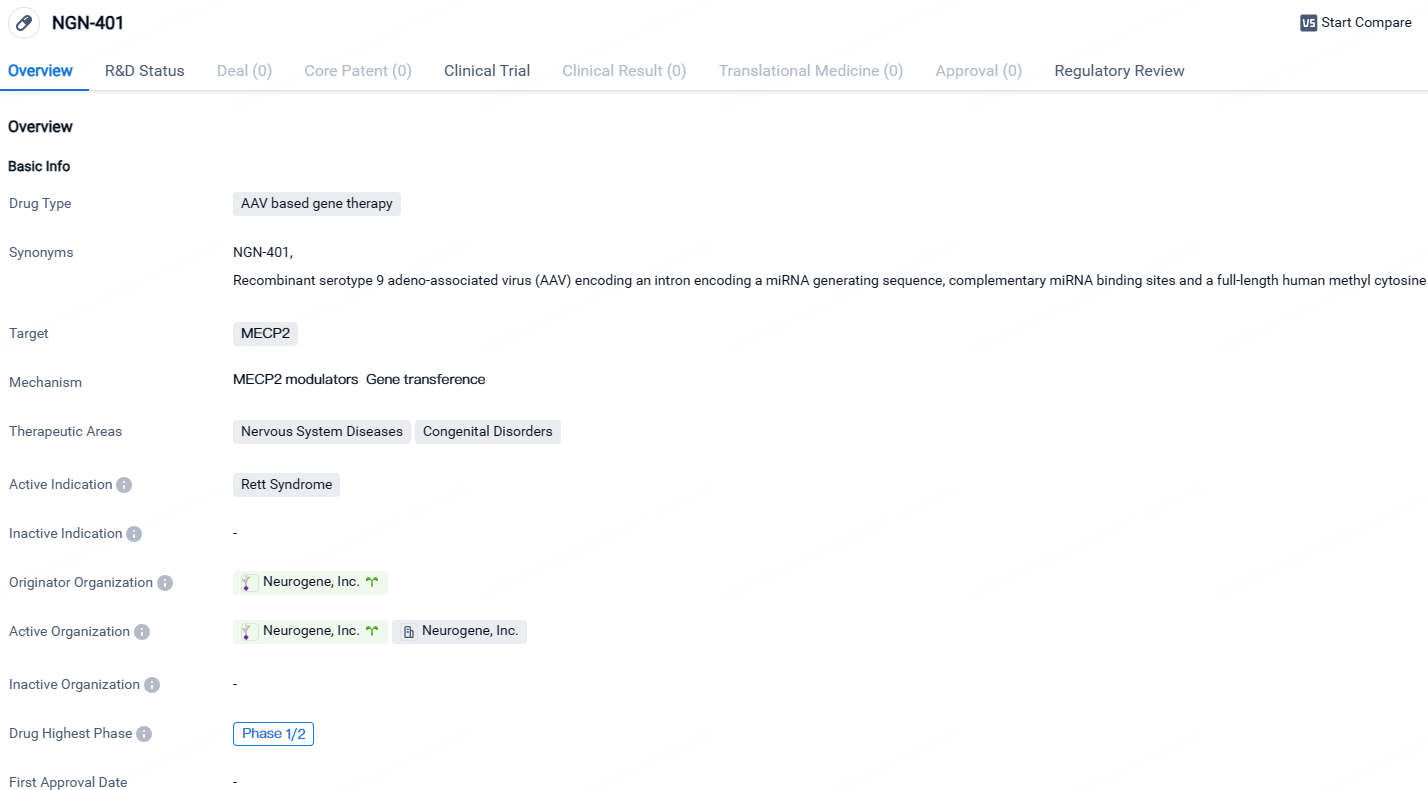
The results indicate that the NGN-401 gene therapy candidate has generally been tolerated well, with no serious adverse events linked to the treatment or procedure, nor indications of toxicity due to overexpression of MeCP2 reported in any of the patients, including one with a mild variant likely to retain some MeCP2 function.
Rachel McMinn, Ph.D., Founder and CEO of Neurogene, commented, “We are eager to present the preliminary but positive safety profile of NGN-401, employing our unique EXACT transgene regulation technology to administer a full-length MECP2 gene specifically to critical brain regions implicated in Rett syndrome’s pathophysiology.” She added, “Rett syndrome presents as a complex neurological disorder with limited treatment leeway. Traditional gene therapy models have struggled to adjust protein levels therapeutically without causing harmful overexpression. Thus, we find it crucial to discuss these safety results at the ASGCT Meeting before our projected interim efficacy results in the fourth quarter of 2024. We now have several months of data from three patients indicating that NGN-401 has been largely well-received.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
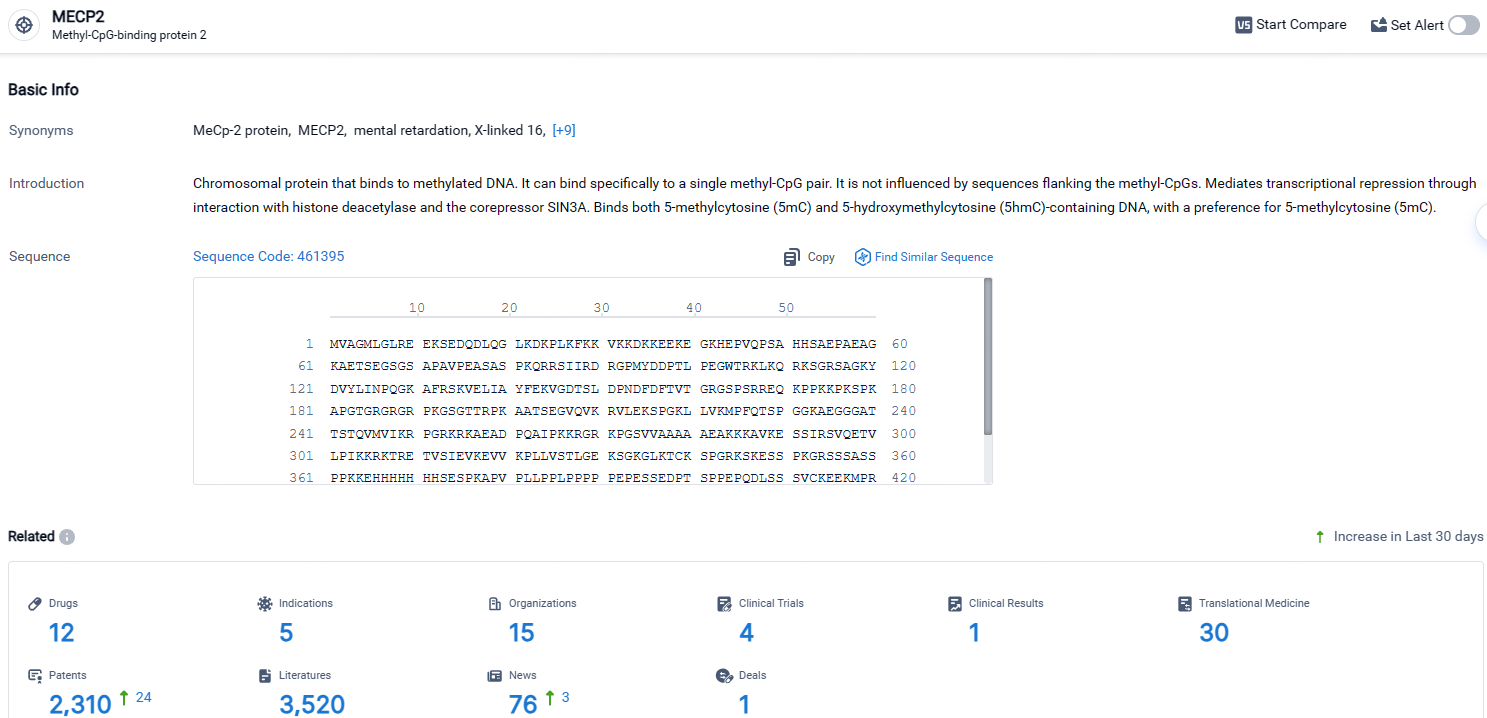
According to the data provided by the Synapse Database, As of April 24, 2024, there are 12 investigational drugs for the MECP2 target, including 5 indications, 15 R&D institutions involved, with related clinical trials reaching 4, and as many as 2310 patents.
NGN-401 shows promise as a potential treatment for Rett Syndrome, a rare congenital disorder affecting the nervous system. Its AAV-based gene therapy approach and targeting of MECP2 indicate a novel and targeted therapeutic strategy. The drug is currently in Phase 1/2 of clinical development, and its regulatory designations as a Rare Pediatric Disease, Fast Track, and Orphan Drug highlight its potential to address unmet medical needs and provide significant benefits to patients. Further research and clinical trials will be necessary to determine the safety and efficacy of NGN-401, but it represents a potential breakthrough in the treatment of Rett S.