FDA Grants Approval for Likmez (metronidazole) Liquid Form to Treat Parasitic and Anaerobic Bacteria-Related Conditions
Canadian biopharmaceutical firm Appili Therapeutics Inc., which is primarily dealing with creating drugs for infectious diseases and biodefense, released a statement that Saptalis Pharmaceuticals, its partner for production and marketing, has been granted authorization by the U.S. FDA for Metronidazole Oral Suspension 500mg/5mL.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Appili's ATI-1501, a liquid oral form of the antibiotic metronidazole, has been authorized to Saptalis for sale in the U.S., and other select regions. The FDA also gave the green light to the brand name Likmez™ for ATI-1501.
Don Cilla, Pharm.D., M.B.A., CEO and President of Appili, stated that the FDA's blessing of Likmez, soon after obtaining patent rights unto 2039, is a notable achievement showcasing Appili's proficiency at recognizing potential targets and advancing them while simultaneously benefiting patients and stockholders. He emphasized that patients facing issues with swallowing tablets should not lack access to appropriate antibiotic treatment. Hence, Likmez serves as an accessible alternative for those who struggle to consume solid oral drugs.
Mr. Cilla further stated that forming Appili was primarily for the development of ATI-1501, and it's heartening to witness our debut development task hit the market. It's indeed a magnificent team success and they are eager to offer the product to patients soon and look forward to unleashing more products in the market that solve grave global issues in communicable diseases.
Polireddy Dondeti, Ph.D., CEO and President at Saptalis expressed his pleasure at the approval of the NDA for Likmez. He is proud his team's success in using Appili’s taste masking technology to create a commercial product that the FDA accepts. He conveys that Likmez will meet a definite market requirement in a more user-friendly dosage form of metronidazole, and they are ready to deploy marketing and distribution strategies in the upcoming days.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
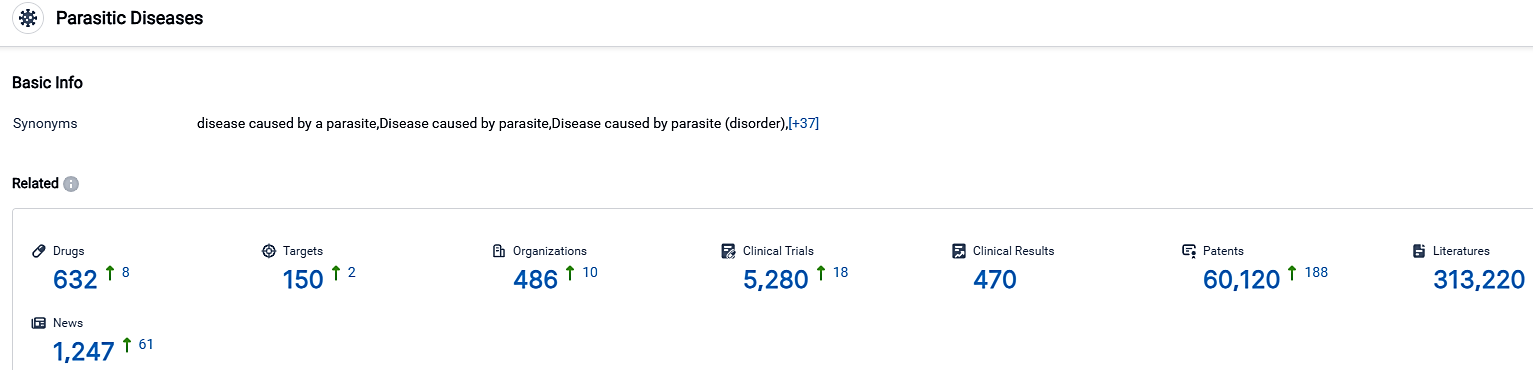
According to the data provided by the Synapse Database, As of September 29, 2023, there are 632 investigational drugs for the Parasitic Diseases, including 150 targets,486 R&D institutions involved, with related clinical trials reaching 5280,and as many as 60120 patents.
Metronidazole is a commonly prescribed oral remedy for parasitic and anaerobic bacterial infections, with an annual prescription count exceeding 10 million in the United States alone. Despite being the only sanctioned oral variant of metronidazole in the U.S. market, the bitter flavor and unsuitable dosage forms for patients struggling with swallowing, make adhering to the therapy regimen a hurdle.