First Participant Administered in Stage 1b Clinical Evaluation of STK-012, Intended for Therapy of Solid Tumors
Synthekine Inc., a pioneer in the field of engineered cytokine treatments, has released information stating that they have concluded the phase 1a dose intensification. Moreover, the initial patient has been treated in the Phase 1b segment of their clinical trial.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
STK-012 is designed specifically to activate antigen-triggered T cells, crucial for powerful anti-cancer effects, while avoiding broader activation of other lymphocytes, such as NK cells related to IL-2 toxicity. Synthekine recently concluded the Phase 1a dose-increment component of the trial in subjects with different advanced solid tumors, with data indicating a distinctive safety profile and single drug effectiveness.
In the Phase 1b segment of the research, dose expansion groups for evaluating STK-012 as a standalone therapy at the recommended Phase 2 dosage in selected solid tumor forms, including NSCLC and RCC.
"IL-2 plays a vital role in activating T cells and enhancing anti-cancer immunity," stated Naiyer Rizvi, M.D., Synthekine's top medical official. "However, no one has managed to decipher the many-sided nature of IL-2 and create a drug that genuinely broadens the therapeutic index. STK-012 is rooted in unique understanding of IL-2 biology and is the first alpha/beta biased IL-2 with clinical results. We've noticed promising standalone effectiveness with STK-012 in dose escalation, and look forward to commencing our cohort enlargement studies."
"High-dosage IL-2 has given long-lasting benefits to patients, even remissions for patients with metastatic kidney cancer and melanoma. Its major toxicity and restricted effectiveness have hindered HD IL-2 from reaching its transformative capability in severe cancers like non-small cell lung cancer," stated David McDermott, M.D., a researcher and head of Medical Oncology at Beth Israel Deaconess Medical Center.
The Phase 1b dose expansion part of the clinical experiment is an open-label, non-randomized multi-center study presently registering participants. This study will continue to assess the anti-cancer activity of STK-012 single therapy and also check safety, tolerability, and pharmacokinetics.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
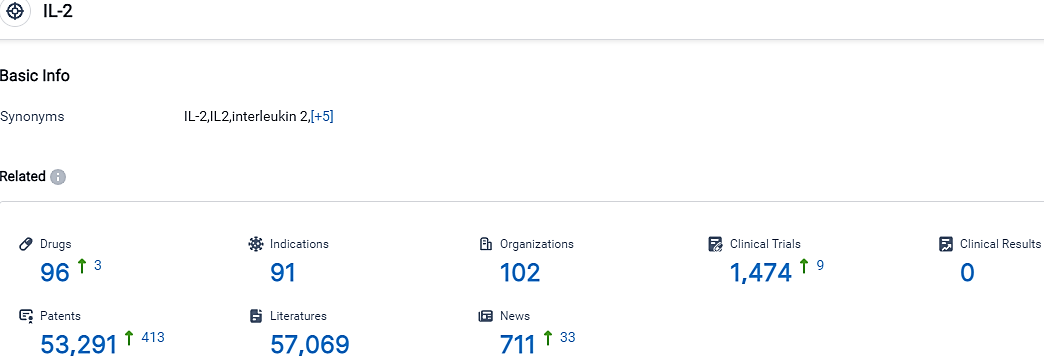
According to the data provided by the Synapse Database, As of October 7, 2023, there are 96 investigational drugs for the IL-2 target, including 91 indications,102 R&D institutions involved, with related clinical trials reaching 1474,and as many as 53291 patents.
STK-012 is a cytokine drug that targets IL-2 and is intended for the treatment of solid tumors. While it shows promise in the field of biomedicine, it is important to note that it is currently in Phase 1 of clinical development, and additional research is needed to determine its effectiveness and safety.