Initial Phase 1 Results of ACE1831: First Anti-CD20 Linked Allogeneic Gamma Delta T Cell Therapy for Non-Hodgkin's Lymphoma
Acepodia, a biotech firm in the clinical phase that is pioneering unique cell therapies through its proprietary Antibody-Cell Conjugation and allogeneic gamma delta 2 T cell technologies, has recently released initial data from the Phase 1 dose-escalation study of ACE1831. This investigational therapy, which consists of anti-CD20 antibody conjugated allogeneic gamma delta T cells, is currently under evaluation for its efficacy in treating individuals diagnosed with non-Hodgkin’s lymphoma.
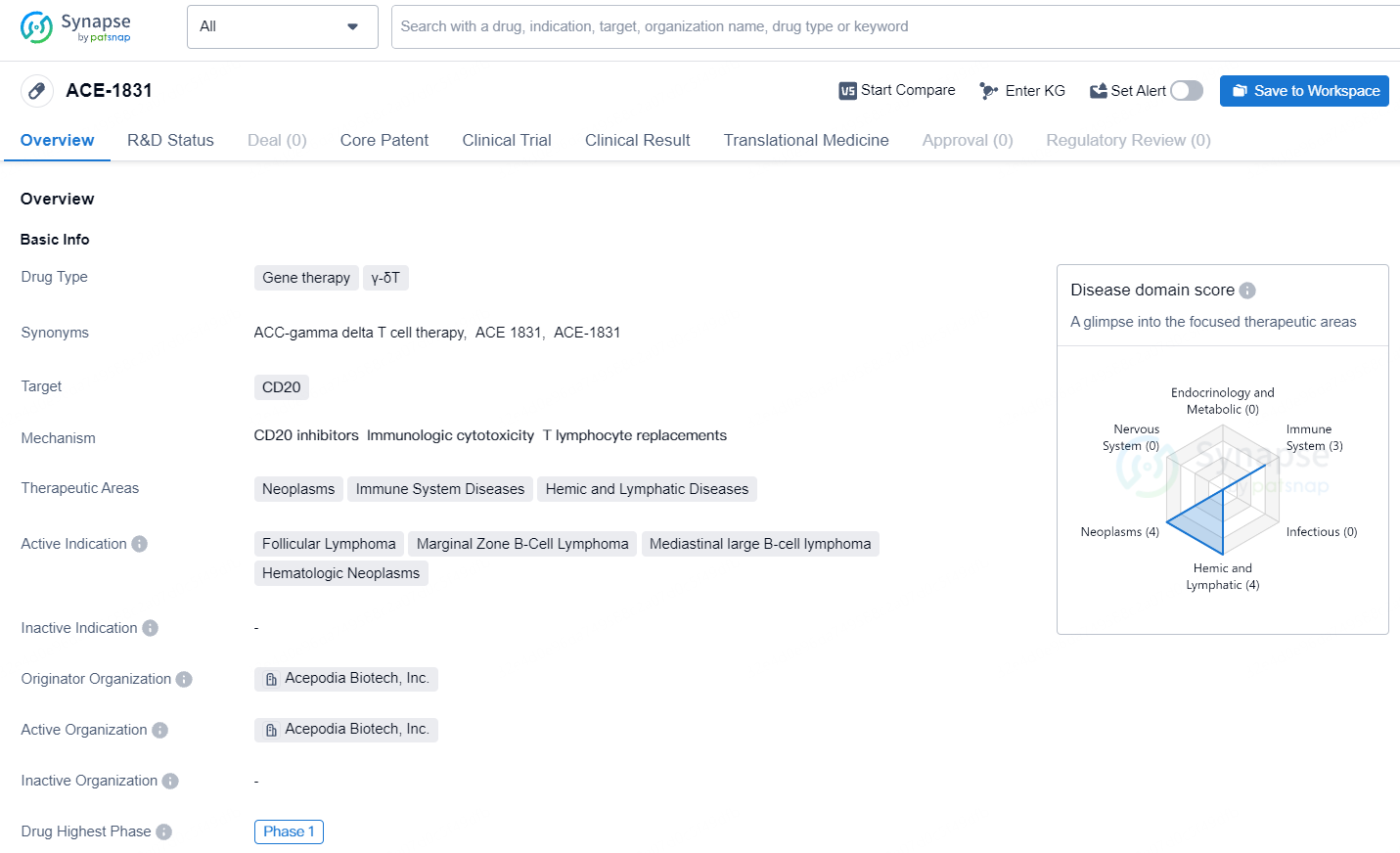
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
In a recent clinical trial, 20% of participants showed a complete response, while 60% achieved disease stability after receiving a minimal dose of ACE1831. Out of these participants, one exhibiting a complete response and two who achieved stable disease had been treated with CD19 CAR-T previously.
Developed by Acepodia, ACE1831 marks the inaugural use of the company's ACC platform in clinical trials. This platform utilizes bioorthogonal chemistry for attaching antibodies against CD20 to gamma delta 2 T cells. Originating from Dr. Carolyn Bertozzi's innovative research in click chemistry on living systems — which garnished the Nobel Prize in 2022 — this approach offers a ready-to-use, non-genetically modified alternative to CAR-T cell therapies, potentially reducing severe side effects like cytokine release syndrome, neurotoxicity, and more.
Initial findings suggest that ACE1831 triggers a proximate and prolonged immune attack against cancer, involving direct cancer cell destruction, coating of the tumor cells (opsonization), and attracting additional T cells via cytokine release, potentially offering broader immune engagement compared to conventional CAR-T treatments.
Sonny Hsiao, Ph.D. and CEO of Acepodia, remarked, "This ongoing trial showcases the therapeutical promises of bioorthogonal chemistry, positioning it as a transformative, inclusive method in cell therapy, particularly when juxtaposed with existing limitations in CAR-T therapies. The encouraging outcomes from the minimal dose point to a significant, enduring effect, emphasizing our commitment to advancing this novel therapeutic strategy."
The initial human, phase 1 assessment of ACE1831 is designed as an open-label, dose-increasing examination to investigate its safety, pharmacokinetics, and effectiveness in individuals with resistant or recurring non-Hodgkin’s lymphoma. This trial, expanding across multiple centers, seeks to include up to 42 participants across the U.S. and Taiwan.
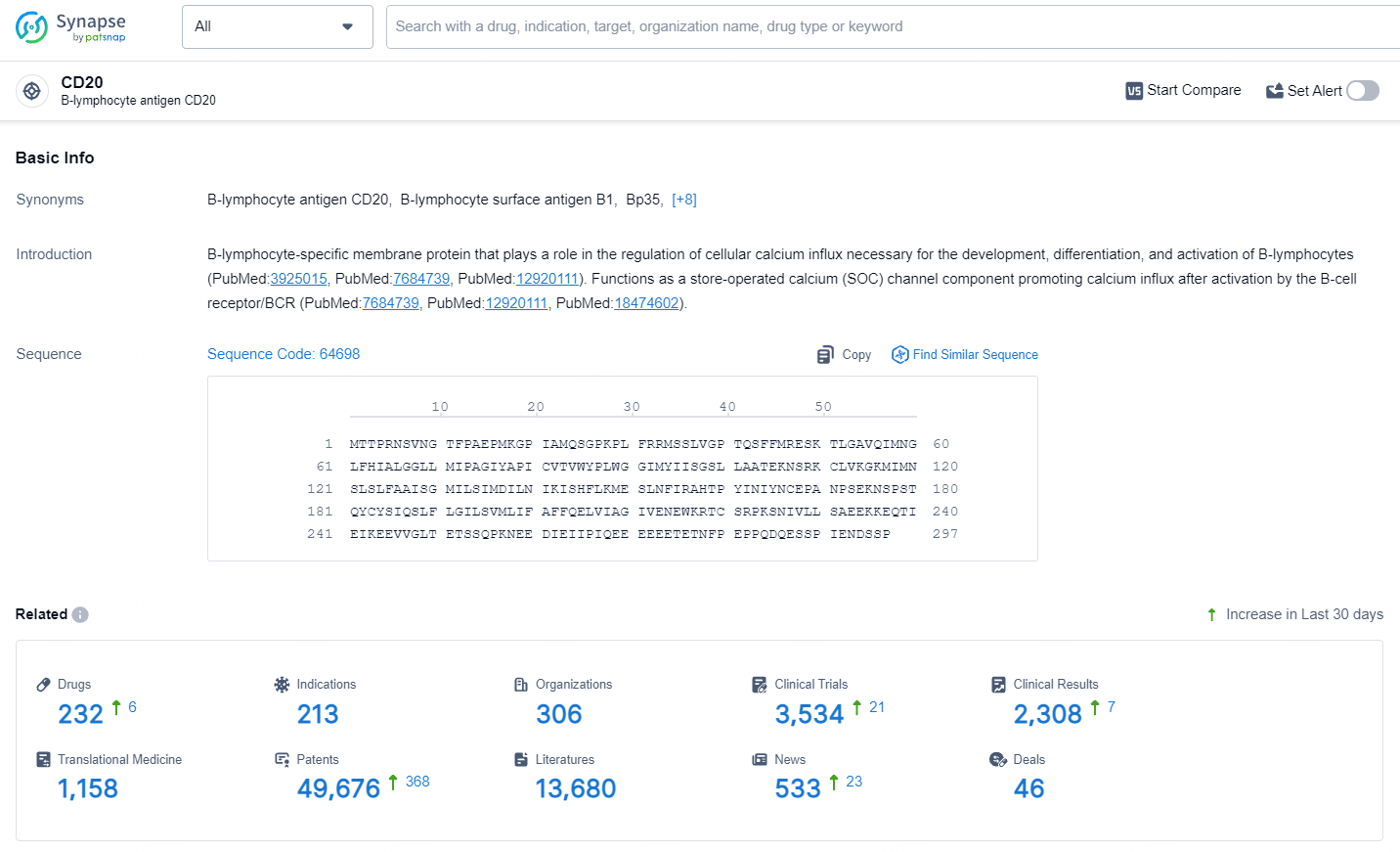
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 11, 2024, there are 227 investigational drugs for the CD20 targets, including 213 indications, 306 R&D institutions involved, with related clinical trials reaching 3534, and as many as 49676 patents.
ACE-1831 targets CD20 and is primarily focused on treating neoplasms, immune system diseases, and hemic and lymphatic diseases. The drug is currently in Phase 1 of development and has shown promise in treating various lymphomas. Further research and clinical trials will be necessary to determine its safety and efficacy in larger patient populations.