Galapagos Unveils Promising Results from CD19 CAR T-Cell Trial in Non-Hodgkin Lymphoma
Galapagos NV has revealed new data from its ongoing Phase 1/2 ATALANTA-1 trial of GLPG5101, a CD19 CAR T-cell therapy. The findings were presented during an oral session at the 66th Annual Meeting and Exposition of the American Society of Hematology (ASH), highlighting a promising efficacy and safety profile in patients with relapsed/refractory non-Hodgkin lymphoma (R/R NHL). The majority of participants in the trial received GLPG5101 as a fresh, fit, stem-like, early memory CD19 CAR T-cell therapy, with a median vein-to-vein time of seven days.
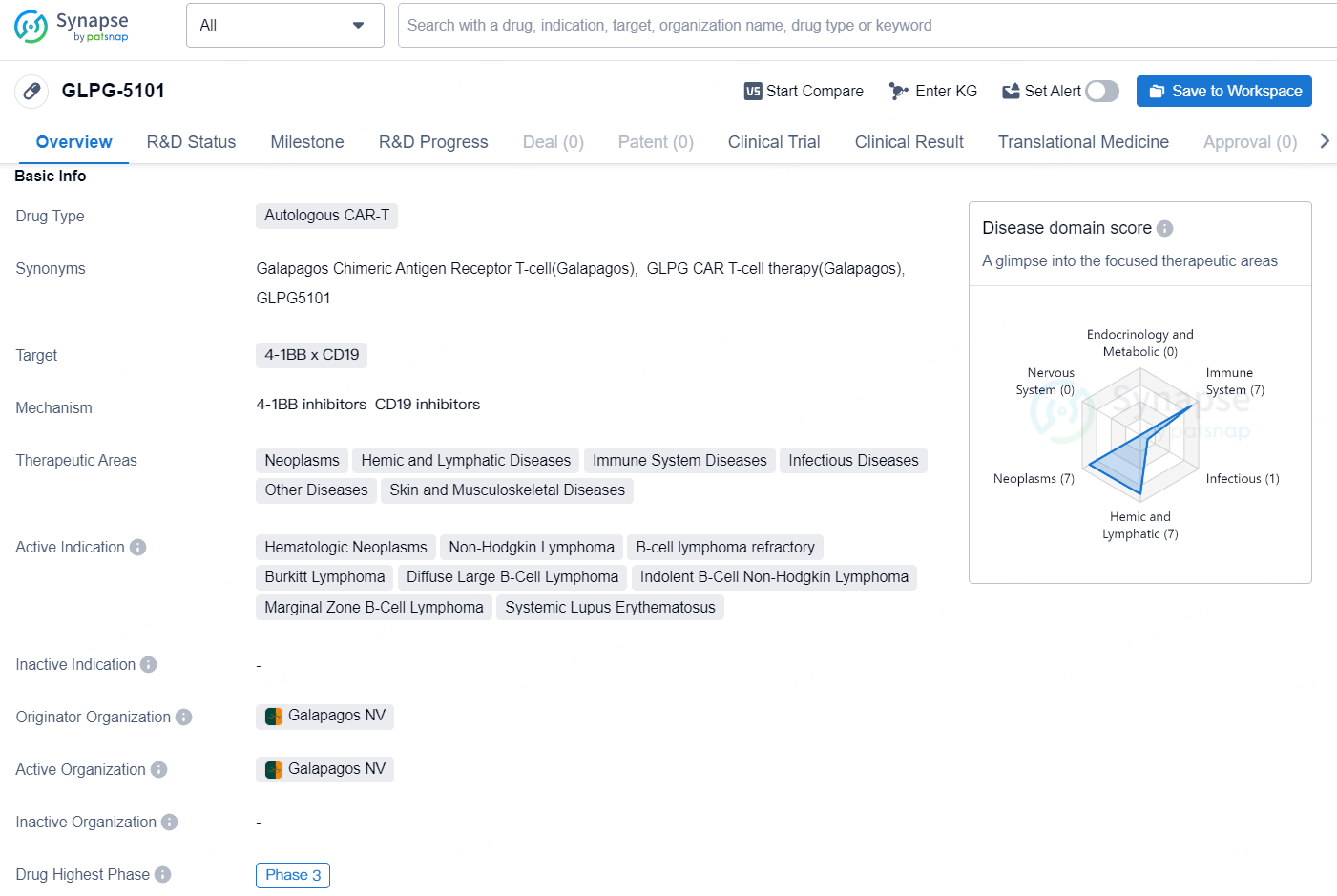
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“Reducing the vein-to-vein time is essential for enhancing patient outcomes and represents a critical unmet need in CAR-T therapy,” stated Marie José Kersten, MD, Principal Investigator for ATALANTA-1 and a Professor of Hematology at the Amsterdam University Medical Center. “The recent findings regarding GLPG5101 are impressive and reveal an encouraging efficacy and safety profile. With an average vein-to-vein time of merely seven days, GLPG5101 holds the potential to provide rapid and flexible treatment scheduling, akin to off-the-shelf therapies.”
“CAR-T therapies are distinctly tailored treatments that currently require a lengthy manufacturing timeline, spanning several weeks to months. For patients dealing with aggressive cancers, every day is crucial, and any delays in treatment can be harmful,” remarked Jeevan Shetty, MD, Head of Clinical Development Oncology at Galapagos. “We remain committed to introducing innovations in cell therapies to tackle the most pressing medical issues. The data we presented at ASH strongly validate the viability of our pioneering decentralized manufacturing approach for cell therapy, which ensures the delivery of fresh, viable cells within a median vein-to-vein time of just seven days, thereby fostering positive patient outcomes.”
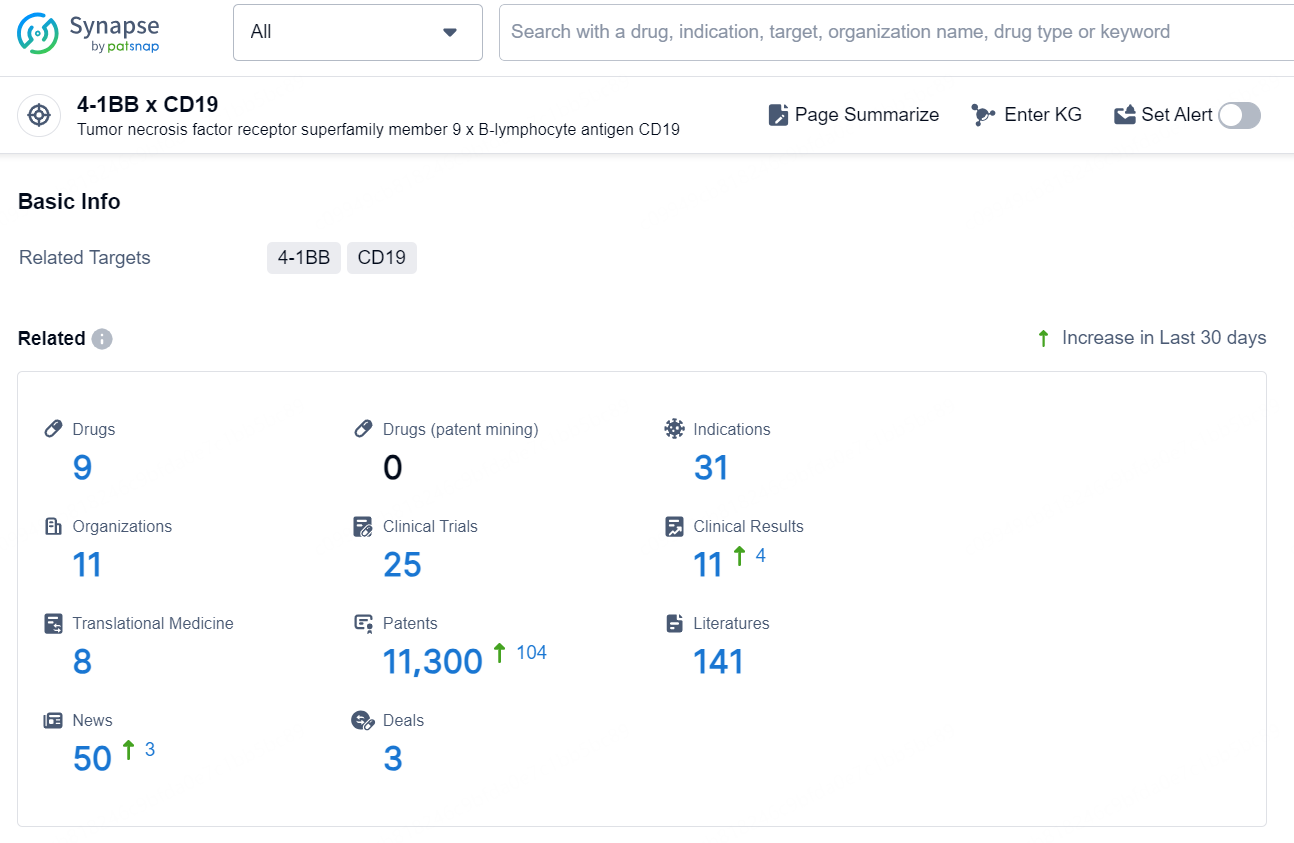
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 11, 2024, there are 9 investigational drugs for the 4-1BB x CD19 target, including 31 indications, 11 R&D institutions involved, with related clinical trials reaching 25, and as many as 11300 patents.
GLPG-5101 is an autologous CAR-T therapy developed by Galapagos NV, which is currently in Phase 3 of development. The drug targets the 4-1BB x CD19 and has a broad therapeutic area, including neoplasms, hemic and lymphatic diseases, immune system diseases, infectious diseases, as well as skin and musculoskeletal diseases.