Trial Shows Epcoritamab Combo Effective for Relapsed/Refractory Follicular Lymphoma
AbbVie (NYSE: ABBV) has released new findings from the Phase 1b/2 EPCORE® NHL-2 study, which examines the efficacy of the investigational drug epcoritamab, a CD3xCD20 bispecific T-cell-engaging antibody delivered subcutaneously. This treatment was evaluated in conjunction with lenalidomide and rituximab (R2) for adults diagnosed with relapsed or refractory follicular lymphoma (FL). Among the 111 participants, observed over a median duration of more than two years, the overall response rate (ORR) reached 96%, while the complete response (CR) rate was 87%. These results (Abstract #342) were presented today during an oral session at the 66th Annual Meeting and Exposition of the American Society of Hematology (ASH).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The findings underscore the promising advantages of epcoritamab for patients suffering from relapsed or refractory follicular lymphoma. Additionally, they indicate that combining epcoritamab with the frequently utilized treatment of lenalidomide and rituximab (R2) may provide a robust and long-lasting therapeutic option," commented Dr. Mariana Cota Stirner, M.D., Ph.D., Vice President and therapeutic area leader for hematology at AbbVie. "These results are very promising as we continue to investigate the efficacy of epcoritamab alongside R2 in an ongoing pivotal Phase 3 trial and aim to affirm its role as a fundamental treatment for B-cell malignancies."
Follicular lymphoma (FL) is generally characterized as an indolent (or slow-progressing) type of non-Hodgkin’s lymphoma (NHL) that develops from B-lymphocytes, accounting for 20-30% of NHL cases, making it the second most prevalent form. Each year, approximately 15,000 individuals in the United States are diagnosed with FL, which is deemed incurable under the current standard care modalities. Patients frequently experience relapses, with each subsequent relapse resulting in a shorter duration of remission and time until the next treatment. Over time, more than 25% of FL patients may experience a transformation to diffuse large B-cell lymphoma (DLBCL), a more aggressive variant of NHL associated with poorer survival rates.
"Given that follicular lymphoma is currently considered incurable, there is a pressing need for additional treatment options for patients with relapsed or refractory cases," stated Dr. Lorenzo Falchi, M.D., a lymphoma specialist at the Department of Medicine, Memorial Sloan Kettering Cancer Center. "The lasting responses observed in the EPCORE NHL-2 trial further endorse the investigation of epcoritamab in conjunction with the standard treatment of rituximab and lenalidomide."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
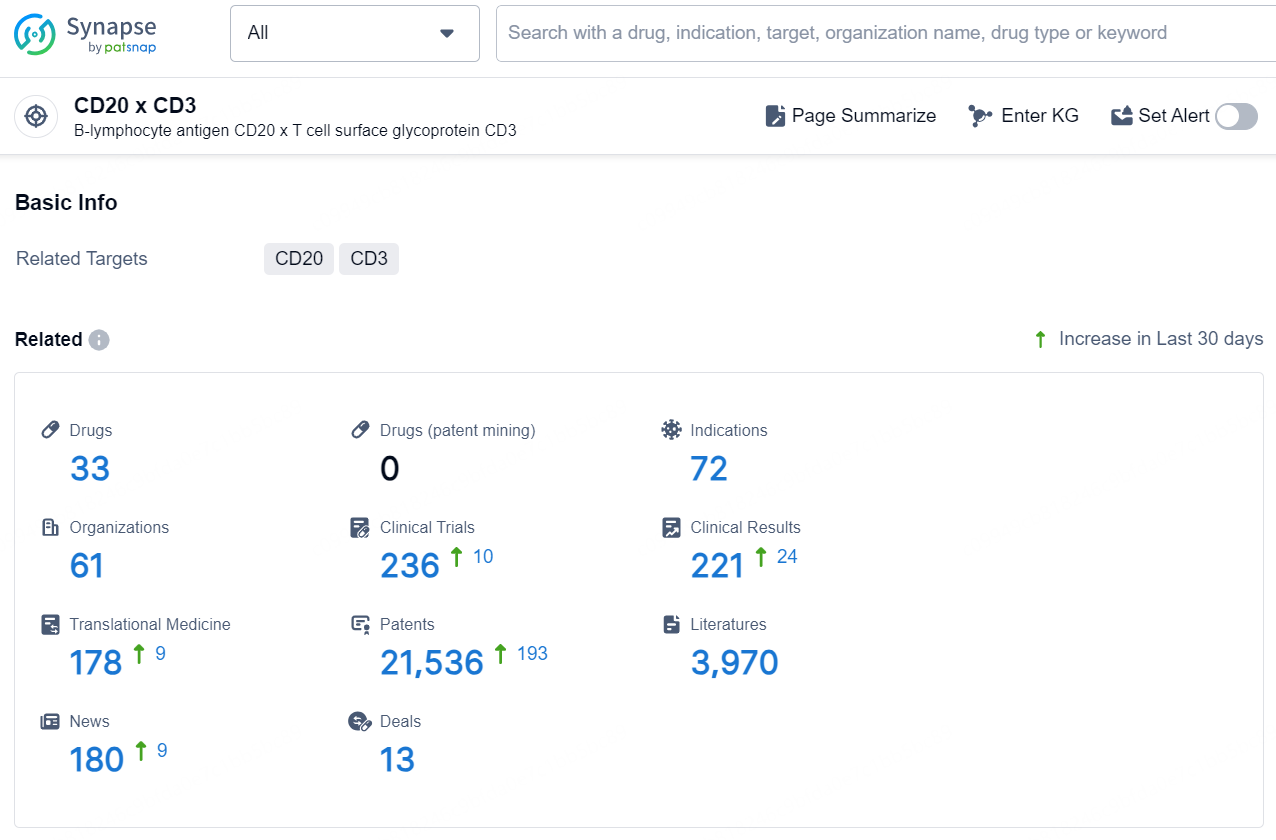
According to the data provided by the Synapse Database, As of December 11, 2024, there are 33 investigational drugs for the CD20 x CD3 targets, including 72 indications, 61 R&D institutions involved, with related clinical trials reaching 236, and as many as 21536 patents.
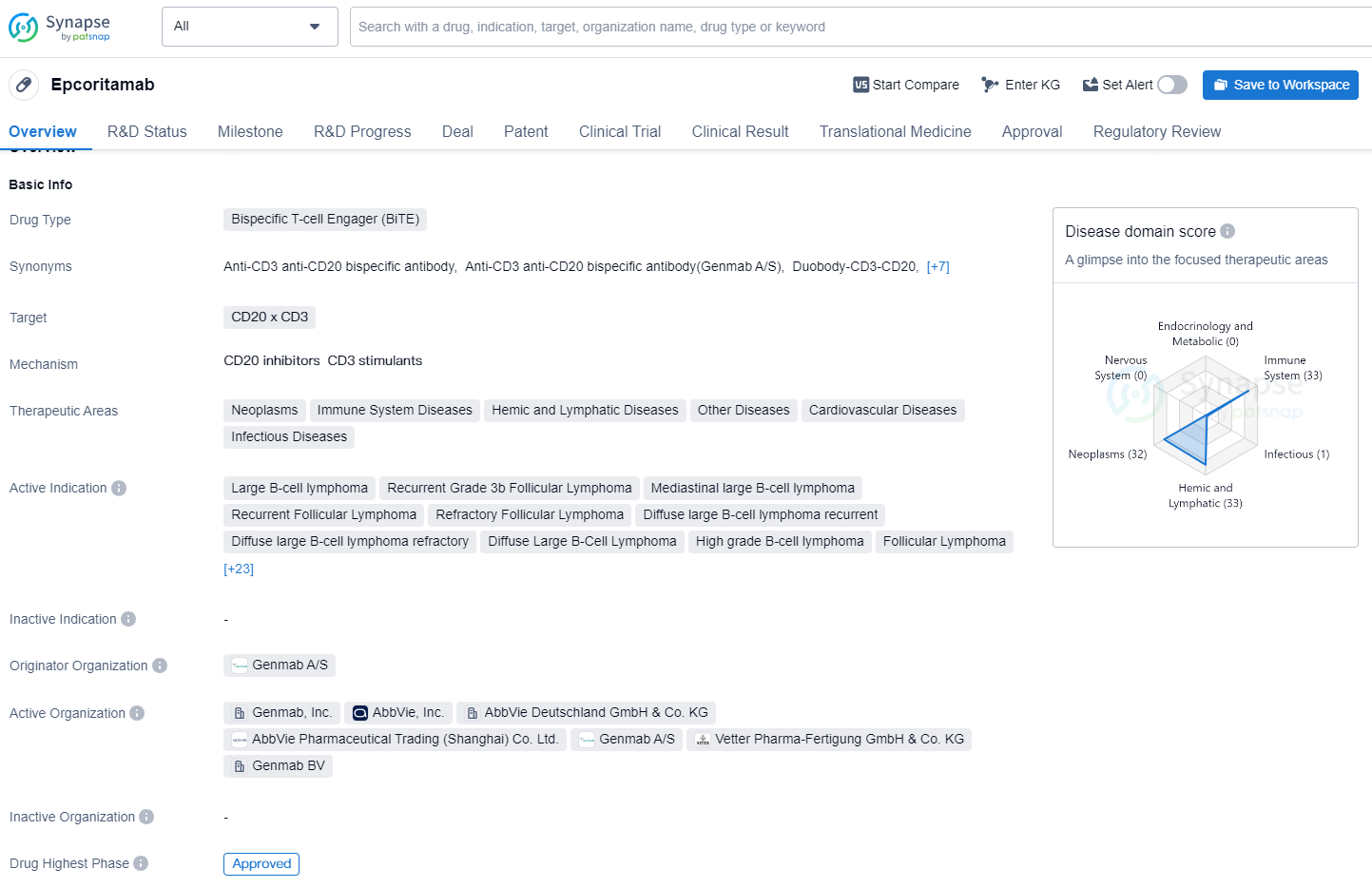
Epcoritamab is a bispecific T-cell engager (BiTE) drug that targets CD20 and CD3. It is designed to treat various neoplasms, immune system diseases, hemic and lymphatic diseases, cardiovascular diseases, and infectious diseases. The drug is indicated for a wide range of conditions including large B-cell lymphoma, follicular lymphoma, mediastinal large B-cell lymphoma, mantle-cell lymphoma, and chronic lymphocytic leukemia, among others.