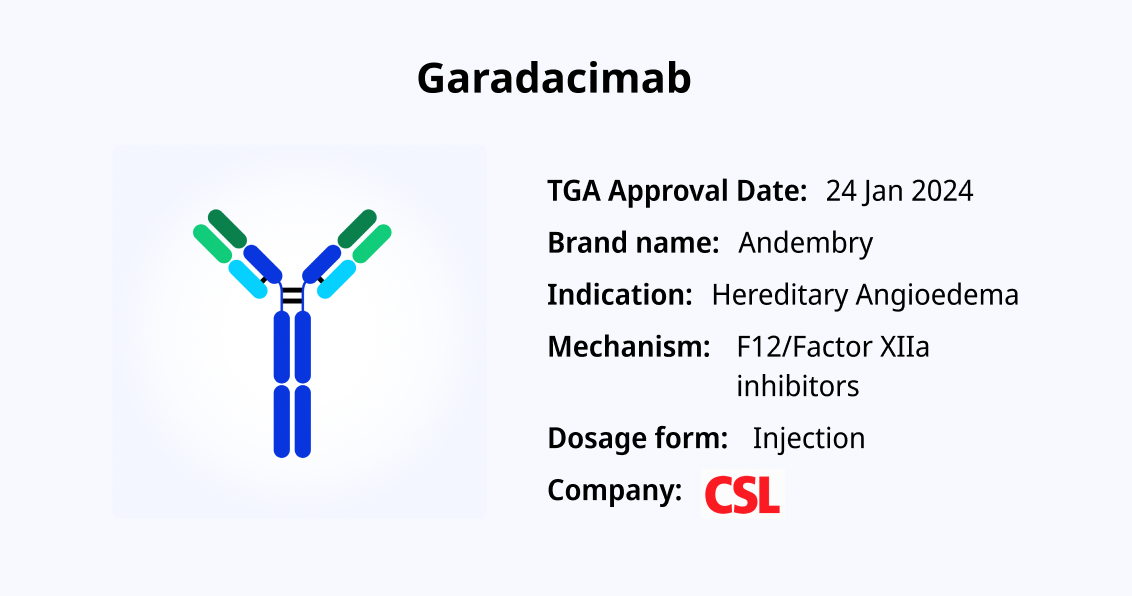
Garadacimab: A Novel FXIIa-Targeting Monoclonal Antibody for Hereditary Angioedema
Garadacimab received its first regulatory approval in Australia on January 24, 2025. It is an innovative, fully human IgG4 monoclonal antibody specifically designed to target activated Factor XII (FXIIa), a key protein involved in blood coagulation and inflammatory pathways. The drug’s mechanism of action involves inhibiting FXIIa activity, thereby reducing the frequency and severity of swelling attacks in patients with hereditary angioedema (HAE).
Mechanism of Action
Hereditary angioedema (HAE) is a rare genetic disorder characterized by recurrent episodes of localized, non-pitting edema in the skin and submucosal tissues. These attacks can affect various body regions, including the extremities, face, genitals, respiratory tract, and gastrointestinal mucosa. During an HAE attack, patients may experience severe abdominal pain, nausea, vomiting, and, in severe cases, life-threatening laryngeal edema.
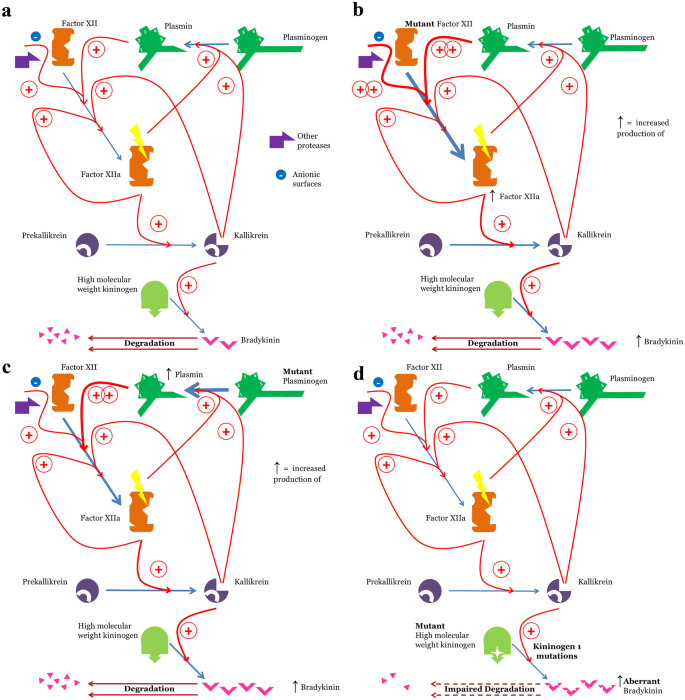
The primary cause of HAE is dysfunction or deficiency of C1 esterase inhibitor (C1-INH), a crucial regulatory protein involved in multiple physiological pathways, including the complement system, fibrinolytic system, and the kallikrein-kinin system (KKS). When C1-INH is deficient or dysfunctional, these systems become excessively activated, particularly the KKS, leading to elevated levels of bradykinin. Bradykinin is a potent vasodilator and permeability-enhancing peptide responsible for increased vascular permeability and subsequent tissue swelling.
Garadacimab exerts its therapeutic effect by selectively binding to and inhibiting activated Factor XII (FXIIa), thereby preventing the excessive activation of the KKS. Under normal conditions, the KKS generates bradykinin through a series of enzymatic reactions. In HAE patients, due to insufficient C1-INH activity, the KKS becomes dysregulated, resulting in excessive bradykinin production and recurrent swelling episodes.
Unlike conventional HAE therapies that target C1-INH or bradykinin directly, Garadacimab intervenes upstream by blocking FXIIa, a critical initiator of KKS activation. By inhibiting FXIIa, Garadacimab effectively reduces the overproduction of bradykinin, preventing the occurrence and progression of swelling episodes.
Drug Characteristics
Following a single administration, Garadacimab provides sustained protection against HAE attacks for up to six months. This prolonged efficacy is particularly beneficial for patients requiring long-term disease management, as it reduces the dosing frequency and may improve patient adherence and quality of life.
In the VANGUARD clinical trial, the majority of patients receiving Garadacimab reported a positive treatment experience, demonstrating a significant reduction in HAE attack frequency and severity. Compared to the placebo group, Garadacimab-treated patients reported noticeable improvements in their overall quality of life. This underscores not only the drug’s clinical efficacy but also its positive impact on patients' daily lives.
As a fully human monoclonal antibody, Garadacimab is designed to minimize immunogenicity risks, suggesting a favorable tolerability profile. Its targeted mechanism and extended duration of action position it as a promising advancement in the preventive treatment of hereditary angioedema.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Refrence
- 1. Wedner HJ. Hereditary angioedema: Pathophysiology (HAE type I, HAE type II, and HAE nC1-INH). Allergy Asthma Proc. 2020 Nov 1;41(Suppl 1):S14-S17. doi: 10.2500/aap.2020.41.200081. PMID: 33109319.