GLP-1R: Therapeutic Target for Obesity and Metabolic Disorders
In exploring the treatment of obesity and its associated complications, such as Type 2 diabetes and cardiovascular disease, a prominent and popular target is the Glucagon-Like Peptide-1 Receptor (GLP-1R). This member of the G protein-coupled receptor family, located on the cell membranes of various tissues, plays a key role in regulating glucolipid metabolism, insulin secretion, and cardiocerebrovascular protection within the body.
The GLP-1 receptor was first isolated in 1992 by researchers from the Drucker laboratory from a rat pancreatic β-cell cDNA library using expression cloning techniques. This receptor consists of 463 amino acids, belongs to the GPCR family, and has been cloned and confirmed in multiple human tissues, including the brain, lungs, and heart.
GLP-1R is widely distributed in tissues such as the islets of Langerhans, brain, heart, kidneys, and gastrointestinal tract.
GLP-1R and Genome-Wide Association Studies (GWAS)
Large-scale, cross-ethnic genome-wide association studies on random glucose levels in 476,326 non-diabetic individuals have identified several genetic loci related to the regulation of random blood sugar, including effects on the GLP1R gene. Among these newly identified loci, rare frequency signals suggest that coding variants in GLP1R may impact the process of GLP-1 mediated glucose regulation.
The low-frequency non-synonymous A316T (rs10305492) variation in the GLP1R coding sequence is associated with lower fasting glucose levels and reduced risk of type 2 diabetes, but it is accompanied by a weakened insulin response to glucose challenge, reduced insulinogenic index, and increased 2-hour postprandial glucose levels. Additional genome-wide association analysis involving 61,488 coronary artery disease patients and 163,728 controls found that the variant at rs10305492 in GLP1R is significantly associated with reduced risk of coronary artery disease, independent of the effect on reduced fasting glucose. Individuals carrying this variant also exhibited trends of decreased LDL cholesterol, triglycerides, and systolic blood pressure, along with increased HDL levels. Moreover, besides being associated with a lower risk of type 2 diabetes, this variant may also provide additional cardiovascular health protection.
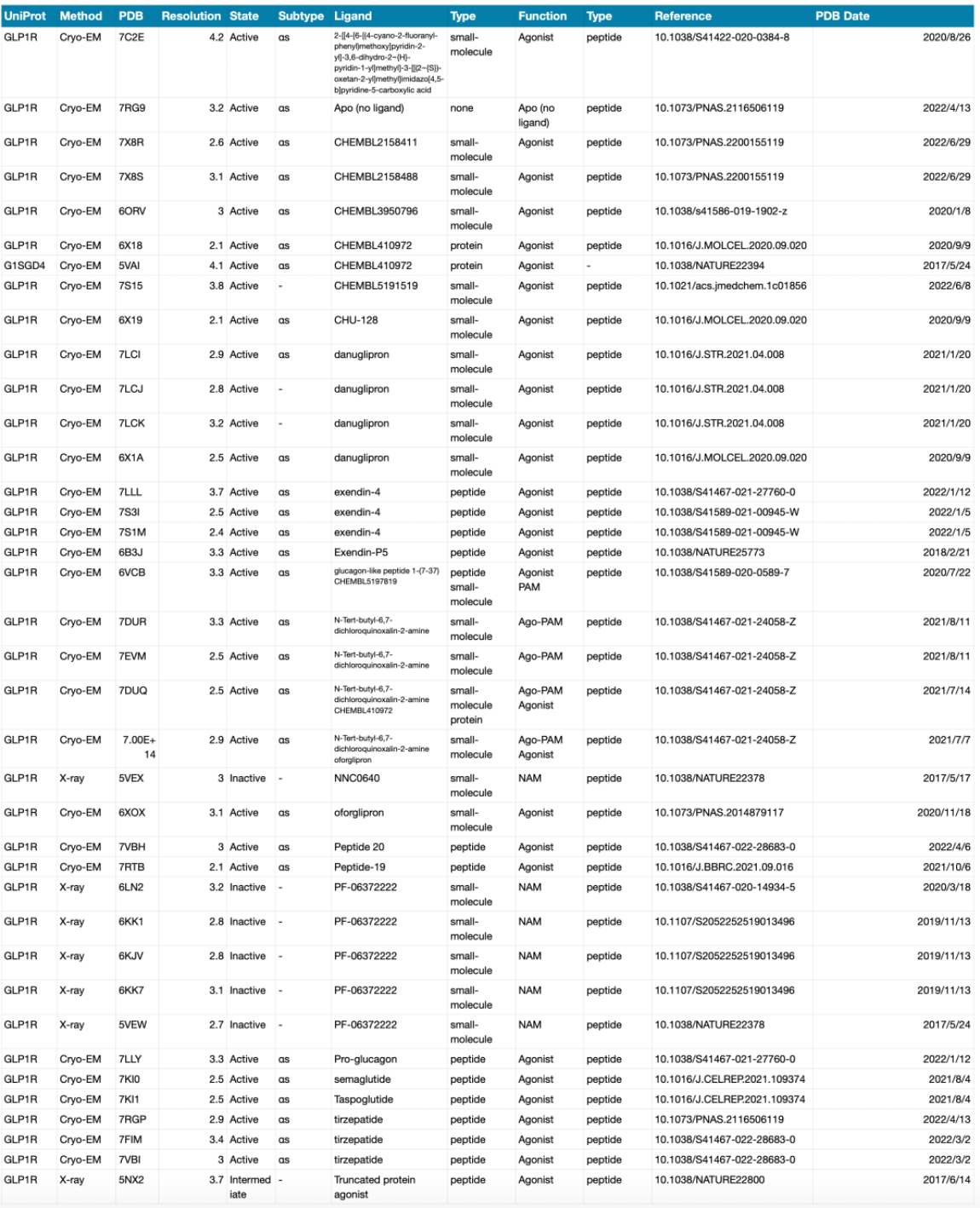
Scientific Progress in Structural Biology
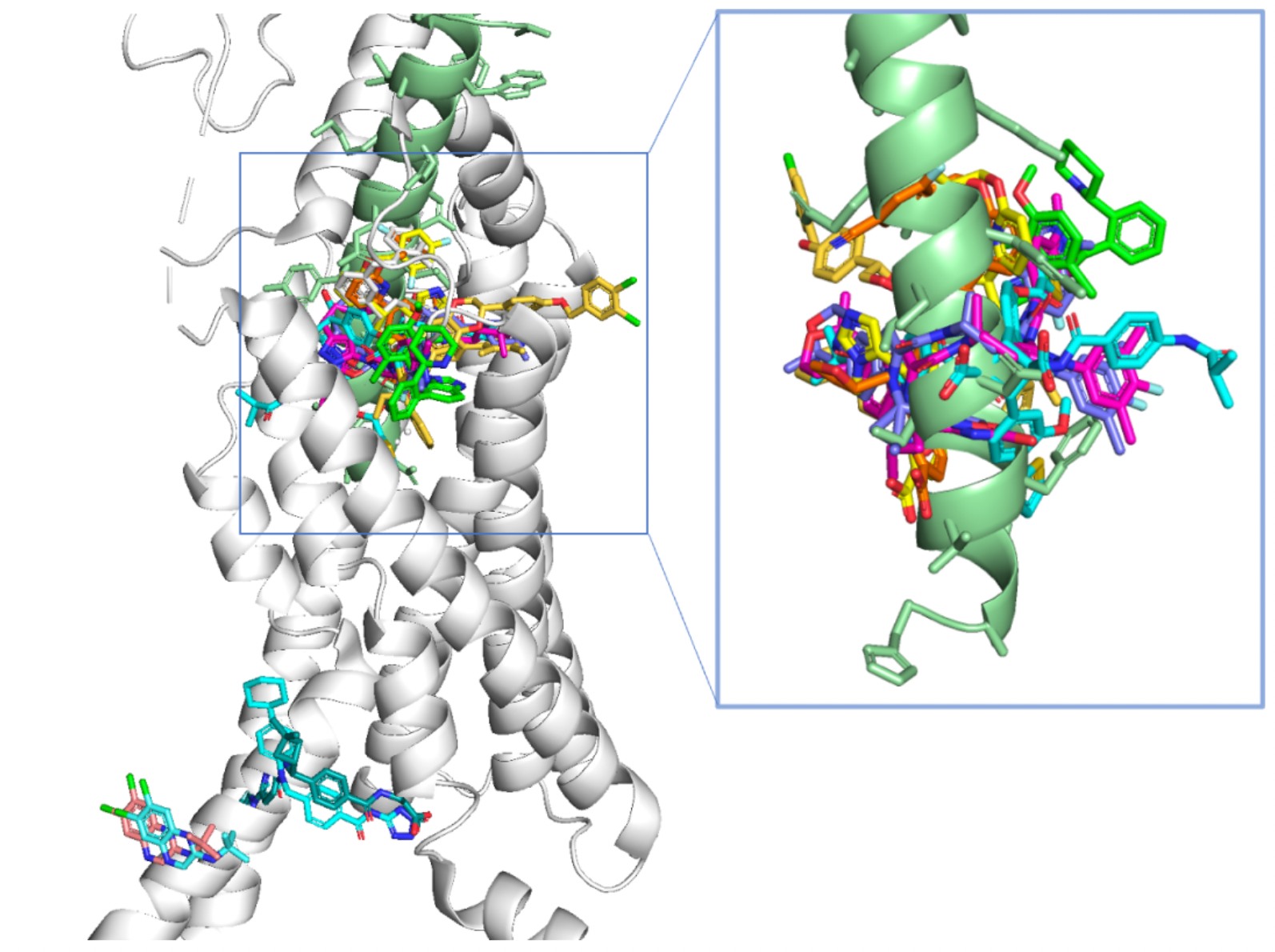
To date, a considerable number of high-resolution structures of the GLP-1R receptor have been elucidated, primarily through the use of cryo-electron microscopy single-particle techniques. The structures of numerous small molecule and peptide-type orthosteric or allosteric ligands have been resolved.
Structural Characteristics of Small Molecule and Peptide Ligands
Based on the structural mechanism studies of GLP-1, Tirzepatide, Danuglipron, LY3502970, and TT-OAD2 involved in GLP-1R receptor recognition, the binding pockets for Danuglipron, LY3502970, and TT-OAD2 are distinctly different: only three residues interact with all three compounds, thirteen residues interact with two of the three compounds, and twenty-two residues only interact with one of the compounds.
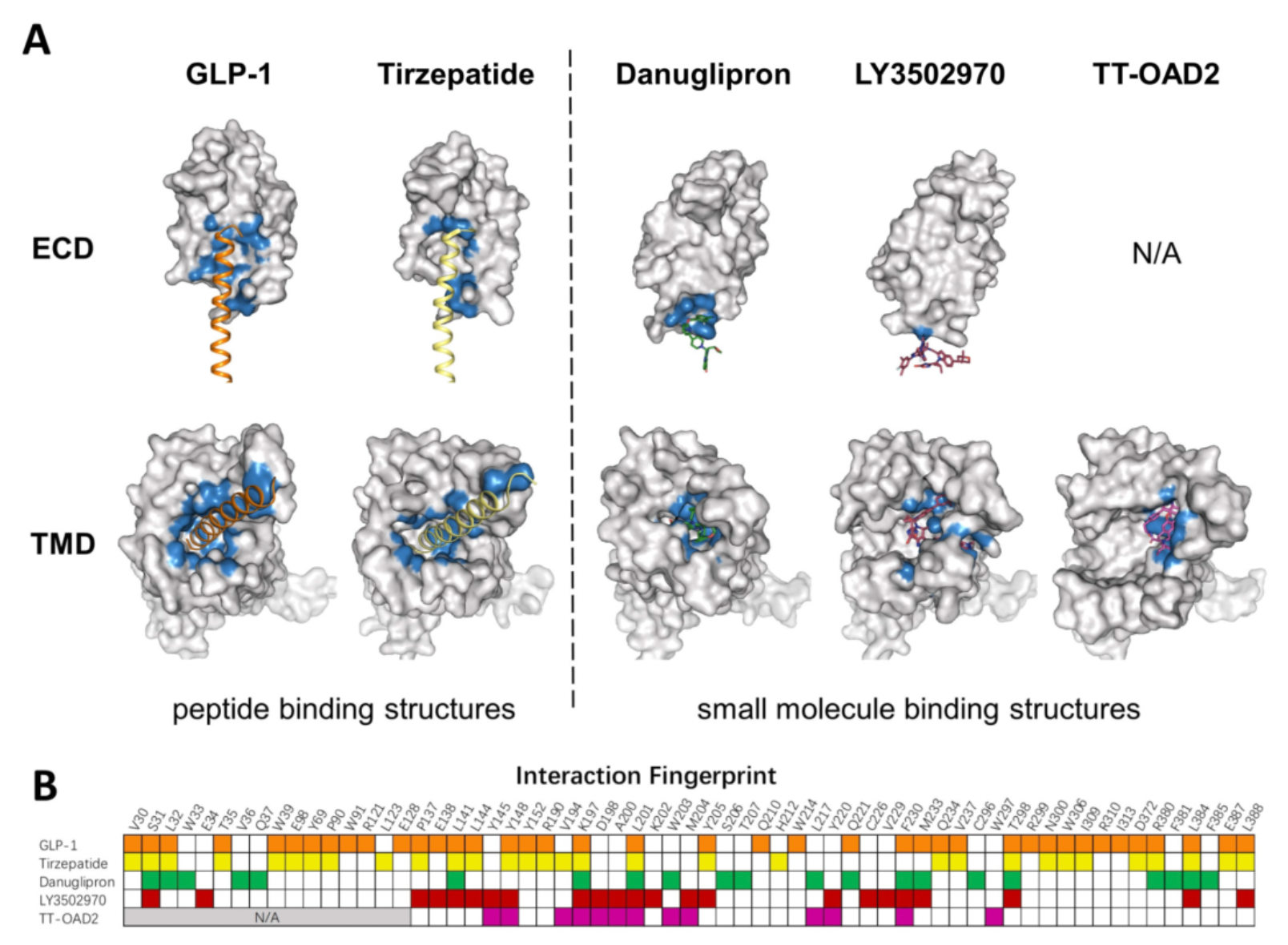
The figure below summarizes that, to date, the structures of eight different small molecule agonists have been resolved, all of which are located near the extracellular ligand-binding pocket compared to the GLP-1 peptide. The N-terminal 7His and 8Ala of the GLP-1 peptide are located in a deeper position within the ligand-binding pocket.
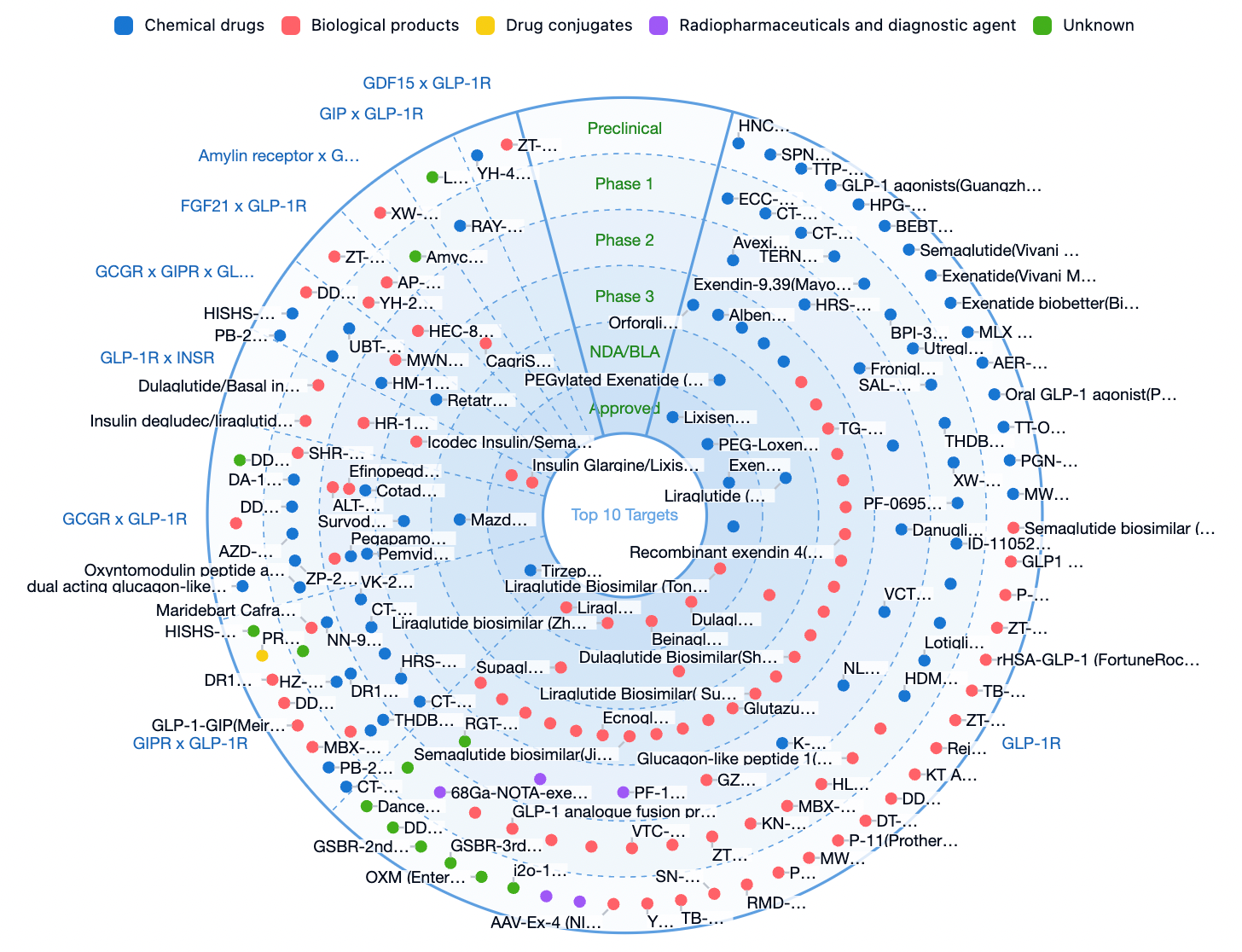
Global GLP-1R Related Pipeline
The pipeline data below are compiled from the Synapse database: In addition to single GLP-1R targets, GLP-1R combined with multi-target therapies has become a major direction in clinical development.
Clinical Treatment of Obesity and Diabetes with GLP-1R
Given the extensive physiological functions and drug development potential of GLP-1R, targeting GLP-1R has become one of the important approaches in treating obesity. GLP-1R agonists such as semaglutide, tirzepatide, and mazdutide not only promote insulin secretion, suppress appetite, and delay gastric emptying, thereby effectively reducing body weight, but also help improve lipid profiles and reduce the risk of cardiovascular diseases.
Innovative therapeutic strategies based on GLP-1 receptor agonists (GLP-1RAs), such as tirzepatide, fixed-ratio combinations of GLP-1RA with basal insulin, combinations of GLP-1RA with sodium-glucose cotransporter-2 inhibitors (SGLT-2i), and high-dose GLP-1RAs, have been listed as one of the most effective options for the treatment of type 2 diabetes.
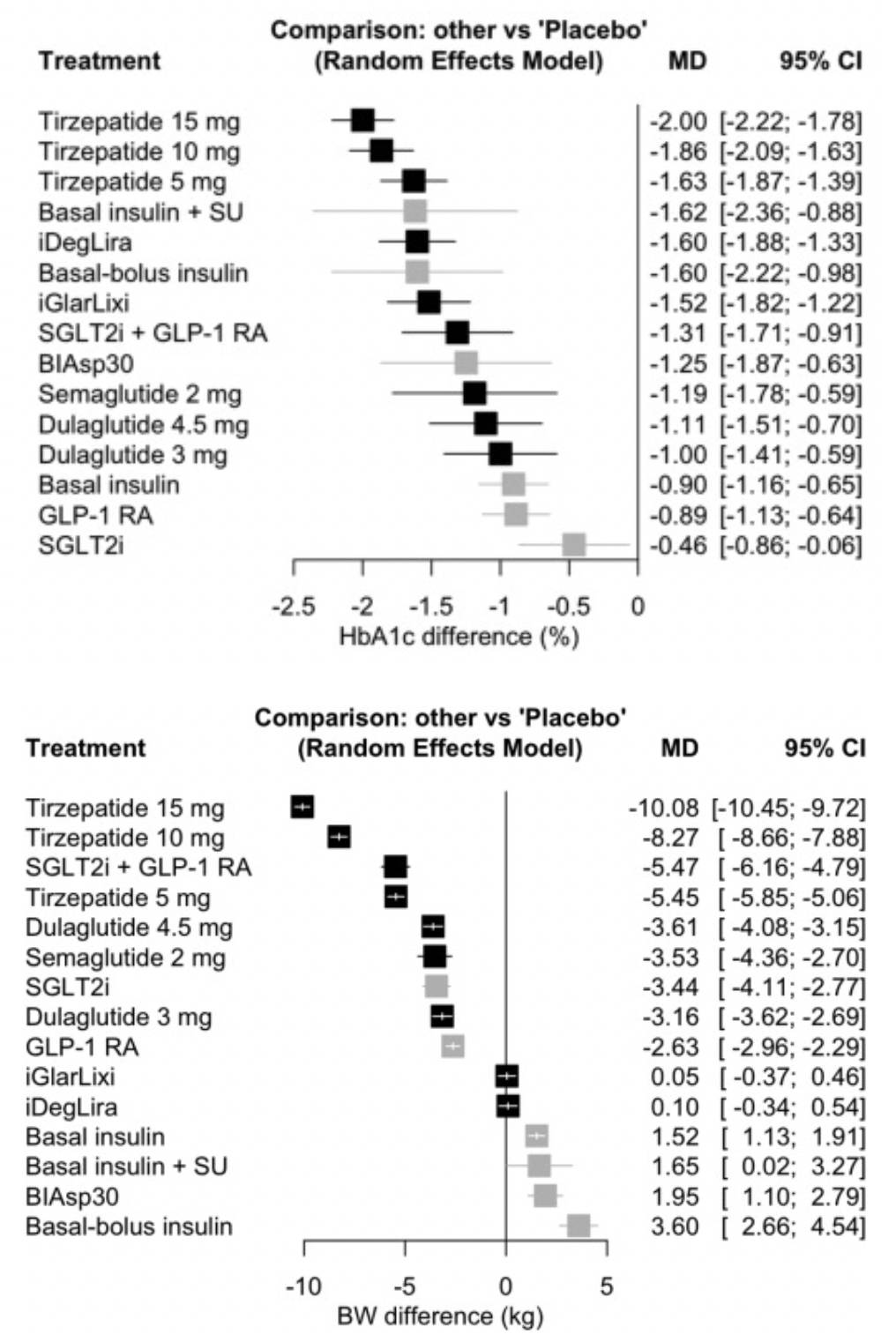
In a systematic review article that included 40 clinical trials, tirzepatide at 15 mg ranked first in terms of HbA1c reduction rate, compared to other GLP-1RA-based strategies, and even those including insulin. Even when compared with high-dose GLP-1RAs (such as semaglutide 2 mg) and combinations of GLP-1RA with SGLT-2i, 15 mg of tirzepatide was also found to be the most effective in reducing body weight.