OncoNano Medicine begins Phase 1 trial of ONM-501, first patient dosed for advanced solid tumors and lymphomas
OncoNano Medicine, Inc. has reported the initiation of treatment for the initial participant at University of Pittsburgh Medical Center as part of their Phase 1 clinical trial. The investigation revolves around ONM-501, an immuno-oncology agent that functions as a dual-activating polyvalent STING agonist. The drug is combined with OMNI™, OncoNano’s proprietary immune activating polymer technology.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
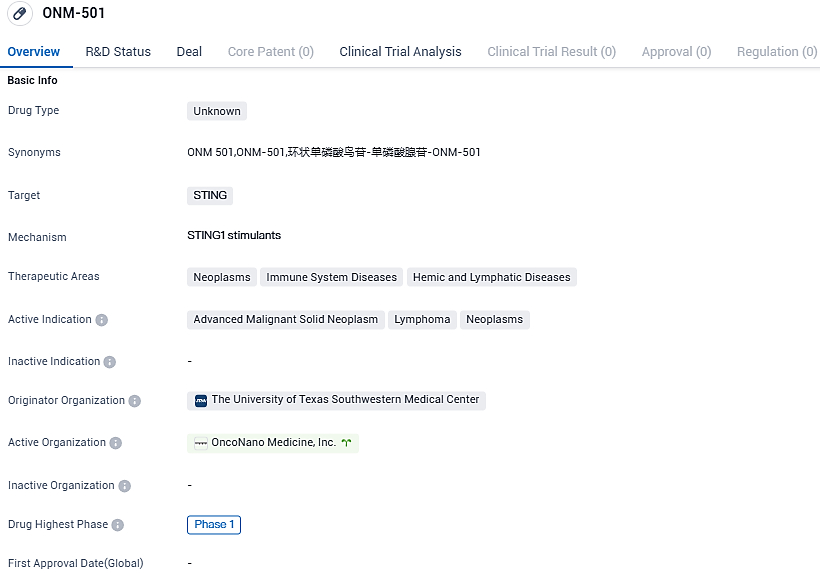
The ONM-501 examination is a phase 1 trial at various medical centers focusing on dose incrementation and spread, conducted on patients struggling with invasive solid tumors and lymphomas. The unique feature of ONM-501 lies in its dual STING activation, utilizing polymers to build micelles, which not only binds to but also activates STING along with the critical payload required for activation. It forms the fabricated copy of the natural ligand cGAMP.
In the stages of combined dose ramp-up and extended cohort of the study, ONM-501 will be administered jointly with Regeneron’s Libtayo® (cemiplimab-rwlc), an anti-PD-1 monoclonal antibody. A collaboration concerning clinical trial supplies for the ONM-501 study has been established between OncoNano and Regeneron.
“We have reached a crucial point in the journey of ONM-501's development with the support of preclinical data indicating a sustained STING activation potentially leading to an enhanced clinical profile, surpassing previous cyclic dinucleotide STING agonists," expressed Kartik Krishnan, M.D., Ph.D., the President and R&D Head at OncoNano Medicine.
The leading therapeutic program of OncoNano, ONM-501, signifies a new age of dual-activating STING agonist. ONM-412, encapsulating the highly potent cytokine, interleukin-12 within the ON-BOARD™ polymeric micelle system forms OncoNano's second therapy development program.
Moreover, the use of the ON-BOARD™ platform has diversified towards pegsitacianine, an innovative nanoprobe exhibiting fluorescence characteristics, explored as an instantaneous surgical imaging agent for several cancer surgeries and now proceeding towards a key clinical trial to assist in tumor detection of peritoneal carcinomatosis. Pegsitacianine and ONM-501 development have been facilitated by grants awarded by the Cancer Prevention Research Institute of Texas.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
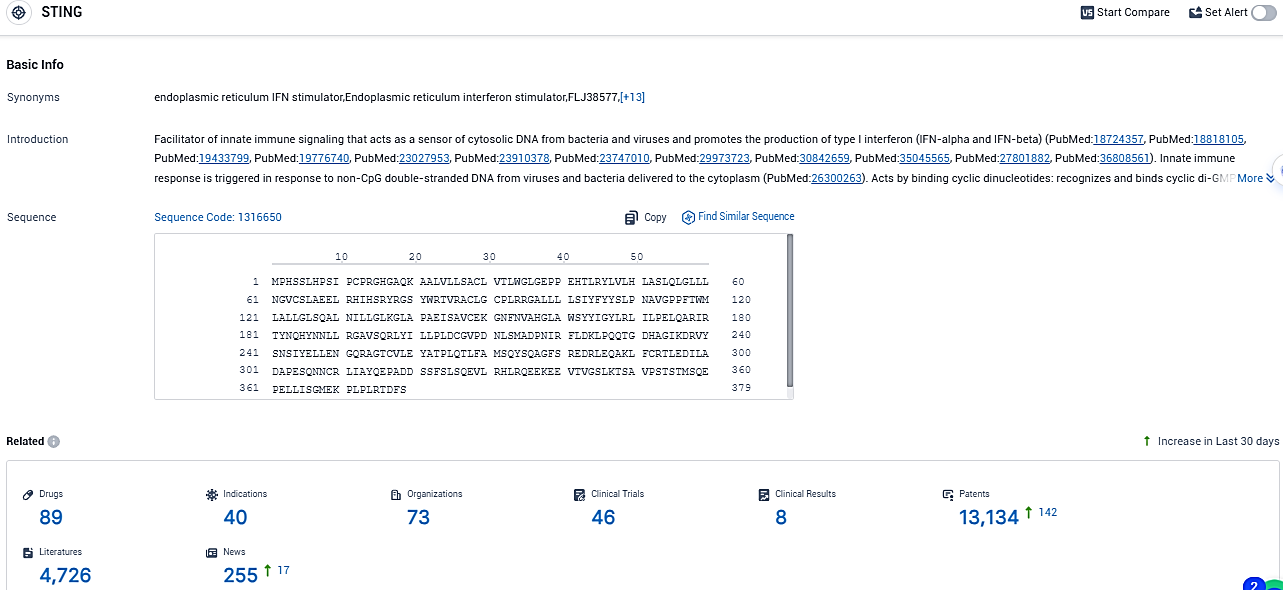
According to the data provided by the Synapse Database, As of November 30, 2023, there are 89 investigational drugs for the STING target, including 40 indications, 73 R&D institutions involved, with related clinical trials reaching 46, and as many as 13134 patents.
ONM-501 is a drug with an unknown type that specifically targets STING. It has shown potential in treating advanced malignant solid neoplasms, lymphoma, and other neoplasms. Currently in Phase 1 of clinical development, further research and testing will be necessary to determine the drug's effectiveness and safety in larger patient population.