Halia Therapeutics Reveals Promising Preclinical Results for HT-6184 and Semaglutide in Obesity
Halia Therapeutics, Inc. (Halia), a biopharmaceutical firm in the clinical stages of development, is progressing new treatments for diseases linked to inflammation. They have disclosed encouraging preclinical outcomes for their experimental drug HT-6184 when used in conjunction with the GLP-1 agonist semaglutide. The research demonstrated notable weight reduction in a diet-induced obesity (DIO) mouse model, showing a synergistic effect that enhances weight loss while preserving lean muscle mass. This approach addresses two vital aspects of obesity management. Lean muscle tissue consumes more energy at rest compared to fat tissue, thus maintaining or increasing lean muscle mass can enhance metabolic rate, promoting more effective and long-lasting weight loss.
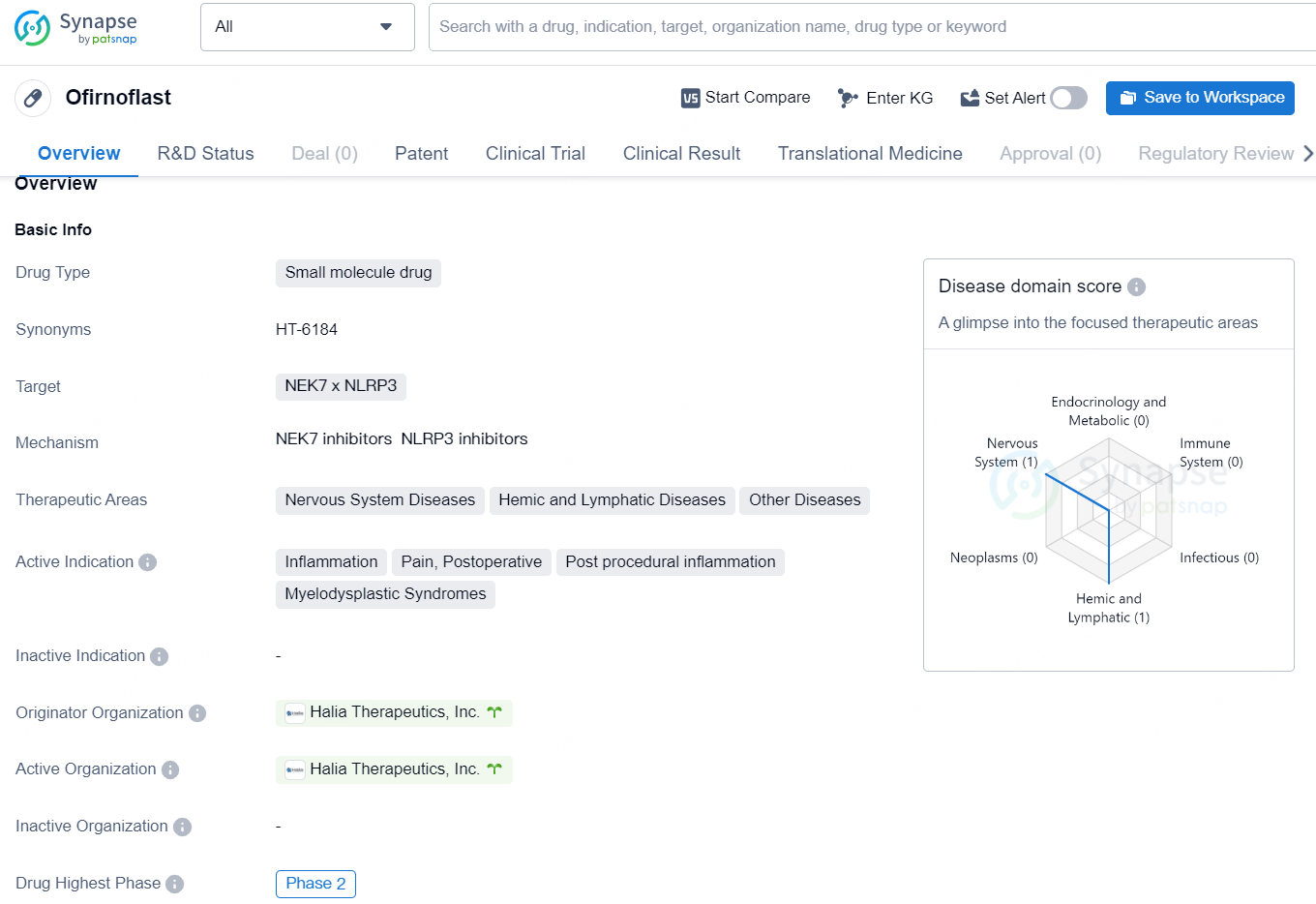
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In a 28-day DIO study, mice administered the combination of HT-6184 and semaglutide showed a significant increase in weight loss. The pairing of HT-6184 with a subtherapeutic dose of semaglutide (1 nmol/kg) led to 50% more weight loss compared to semaglutide alone throughout the duration of the study. When HT-6184 was paired with a higher dose of semaglutide (2 nmol/kg), the weight loss achieved was 25% greater than that with semaglutide alone, surpassing the typical weight loss plateau seen with semaglutide during the 28 days. Moreover, the combination treatment retained lean mass by 10% better compared to the group treated solely with semaglutide by the study’s conclusion.
“These preclinical results are incredibly promising, highlighting HT-6184’s potential as a revolutionary therapy for obesity,” stated Dr. David Bearss, Chief Executive Officer at Halia Therapeutics. “Combining HT-6184 with semaglutide not only enhances weight loss but also preserves lean muscle mass, which is vital for overall metabolic health. These findings bring us a step closer to a more comprehensive treatment for the complex biology of obesity.”
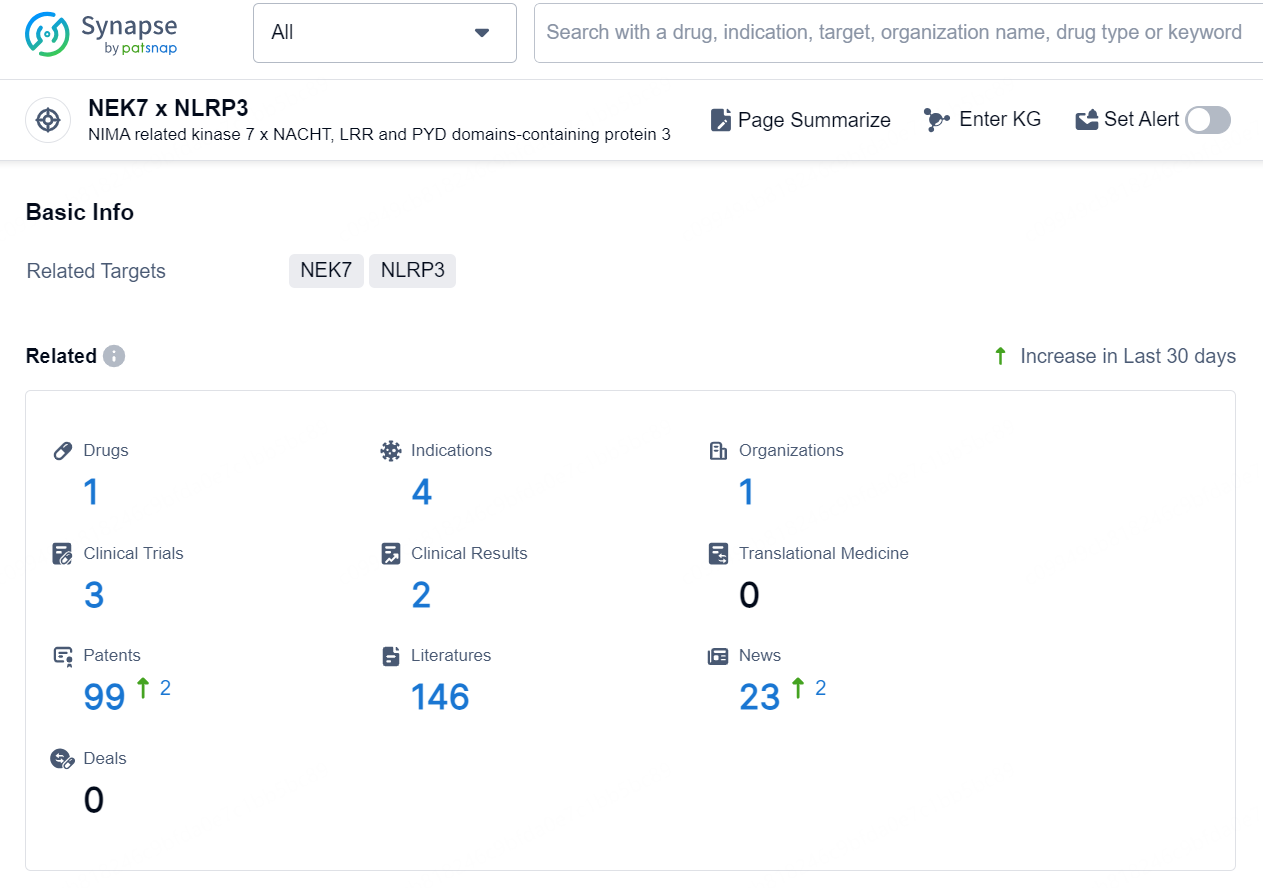
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 24, 2024, there are 1 investigational drug for the NEK7 x NLRP3 targets, including 4 indications, 1 R&D institution involved, with related clinical trials reaching 3, and as many as 99 patents.
Ofirnoflast is a small molecule drug developed by Halia Therapeutics, Inc. It targets NEK7 x NLRP3 and is being developed for the treatment of various therapeutic areas, including nervous system diseases, hemic and lymphatic diseases, and other diseases. The active indications for Ofirnoflast include inflammation, pain, postoperative and post procedural inflammation, as well as the treatment of Myelodysplastic Syndromes.