Hypertension and Metabolic Syndrome Candidate Target: Endothelin Receptor
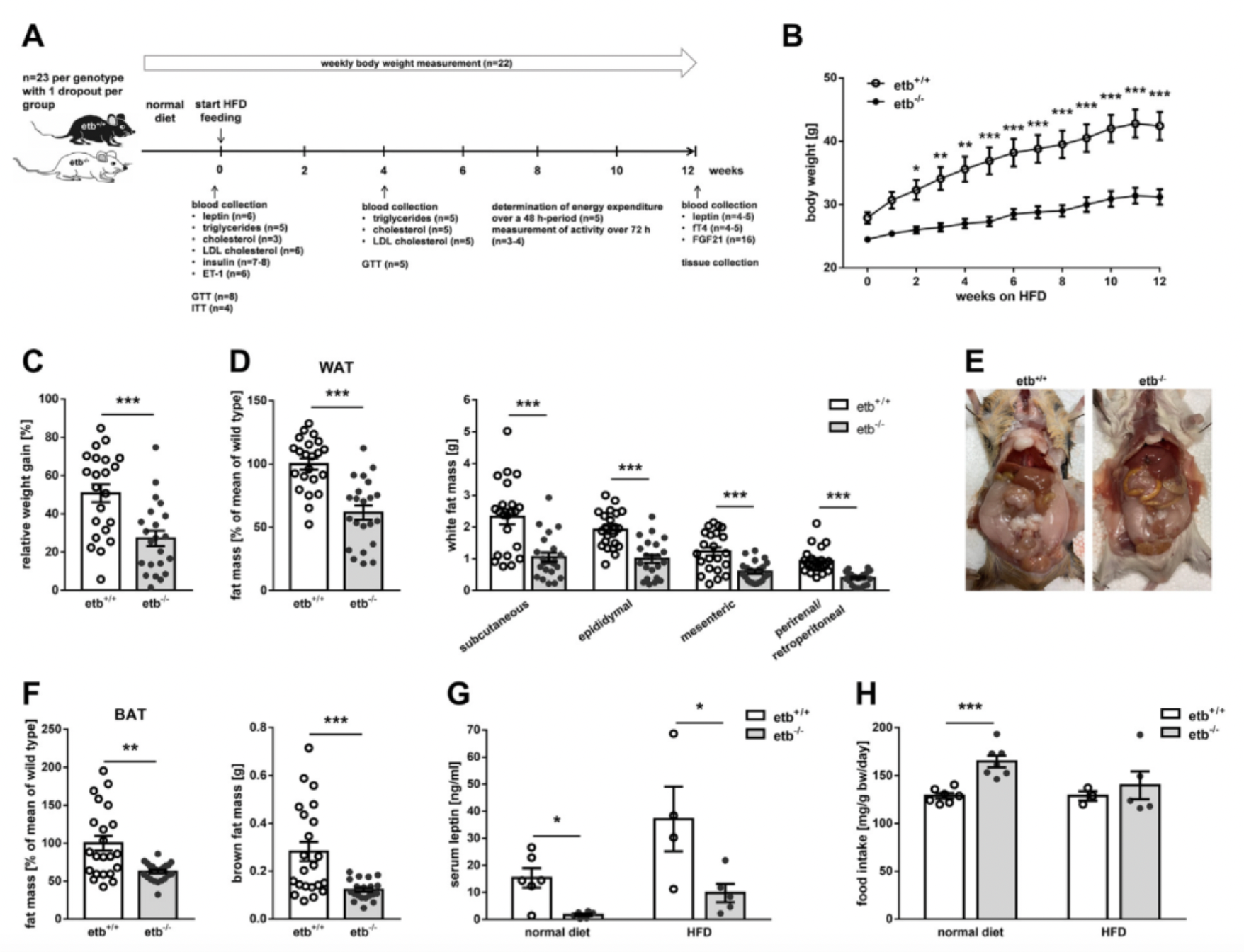
Recently, a collaborative research effort involving multiple German scientific institutions has revealed a critical role for the endothelin B receptor (ETB) in regulating metabolic syndrome induced by high-calorie diets. The original research findings, published in the journal "Molecular Metabolism," indicate that mice lacking the ETB receptors show significant resistance to metabolic syndrome triggered by a high-fat diet.

Worth mentioning is the fact that mice completely lacking ETB receptors can develop Hirschsprung's disease (a fatal condition) due to the absence of ETB in the intestinal nervous system. However, genetically engineered "rescue-type" ETB-deficient mice (those that express a functional ETB in the intestinal nervous system) are viable and can avoid the lethal issues caused by the lack of ETB.
In the model of metabolic syndrome induced by a high-fat diet, ETB-deficient mice displayed better health parameters compared to wild-type mice, such as improved glucose tolerance, increased insulin sensitivity, reduced accumulation of white adipose tissue, lowered serum triglyceride levels, and enhanced energy expenditure. This suggests that the lack of ETB receptors confers a protective effect on mice against metabolic abnormalities caused by high-calorie diets, with their physiological functions not only remaining unimpaired but actually improved.
About Endothelin Receptor
Hypertension is a major threat to global public health. It is estimated to have a prevalence of 46% among adults. Hypertension increases the risk of life-threatening cardiovascular events such as myocardial infarction and stroke. In addition, damage to the brain, heart, and kidneys caused by hypertension can accumulate over time, leading to chronic organ dysfunction.
In 1988, Japanese scientist Yanagisawa and his colleagues reported the discovery of endothelin-1 (ET-1), which sparked a significant interest in the field of biomedicine as a previously elusive "endothelium-derived vasoconstrictor factor."
Endothelin-1 exhibits notable pharmacological properties: it has a long-lasting vasoconstrictive effect on coronary artery smooth muscle, with potency several orders of magnitude greater than angiotensin II or other vasoactive agents. Endothelin-1 is expressed in endothelial cells across all examined vascular beds in humans. A key finding in the first study involving humans was that endothelin-1 maintains normal vascular tone via ETA receptors expressed on smooth muscle. Conversely, ETB receptors, which are expressed by endothelial cells, release the vasorelaxant nitric oxide in an autocrine manner, thus relaxing the underlying smooth muscle.
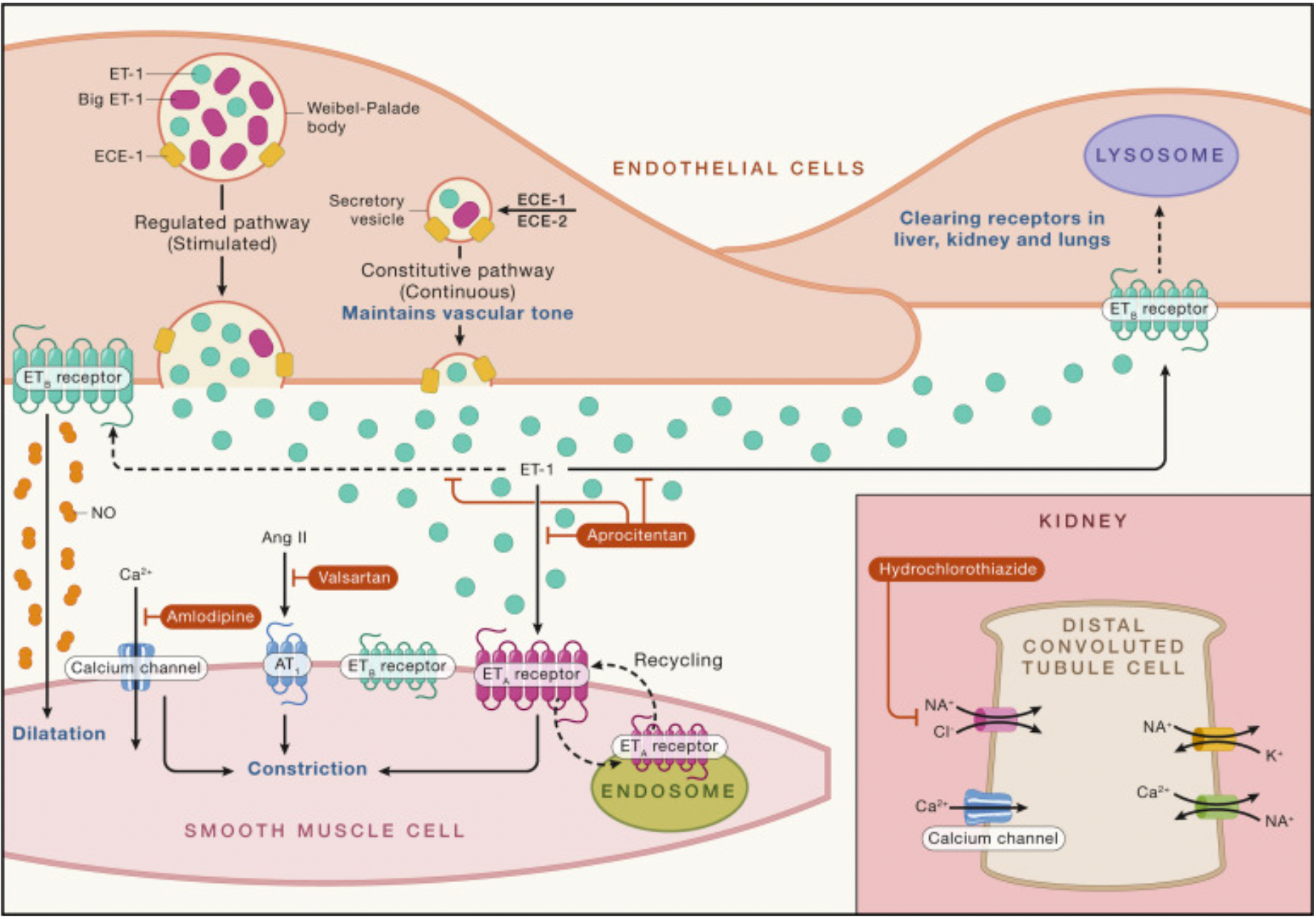
ETA and ETB receptors, as key components of the endothelin system, have significant value in clinical research and therapy. These two G protein-coupled receptors are activated by the endothelin peptides 1-3, which are produced by endothelial cells and a variety of other cells (such as kidney cells, neurons, and macrophages), and are crucial for regulating physiological functions, including the cardiovascular system, kidneys, and metabolism.
In the cardiovascular system, the ETA receptor is primarily associated with vasoconstriction, and its overactivation can lead to increased vascular resistance, thus playing a role in the pathogenesis of diseases such as hypertension and pulmonary arterial hypertension. On the other hand, the ETB receptor promotes vasodilation by stimulating the synthesis of nitric oxide, and in the kidneys, it also participates in the regulation of sodium reabsorption, affecting water and salt balance and blood pressure control.
In clinical applications, antagonists targeting ETA or ETB receptors have been used in the treatment of various diseases, including hypertension, pulmonary arterial hypertension, heart failure, chronic kidney disease, systemic sclerosis, metabolic syndrome, and stroke, among others.
In May 2023, Sovateltide, the world’s first selective ETB agonist, was approved for market release for the treatment of acute cerebral ischemic stroke.
In July 2023, ENB Therapeutics, a biopharmaceutical company focusing on the development of selective antagonists targeting the ETB receptor, announced that its investigational selective ETB receptor antagonist, ETB-003, in combination with Merck's anti-PD-1 therapy, KEYTRUDA® (pembrolizumab), demonstrated positive safety and efficacy data in a Phase 1b clinical trial for patients with refractory advanced solid tumors.
Representative ETB Receptor Clinical Drug Research
Bosentan (Ro-47-0203) is a potent dual antagonist of ETA and ETB receptors, developed by the original research team at Roche in Switzerland, with its activity being studied in various preclinical disease models.
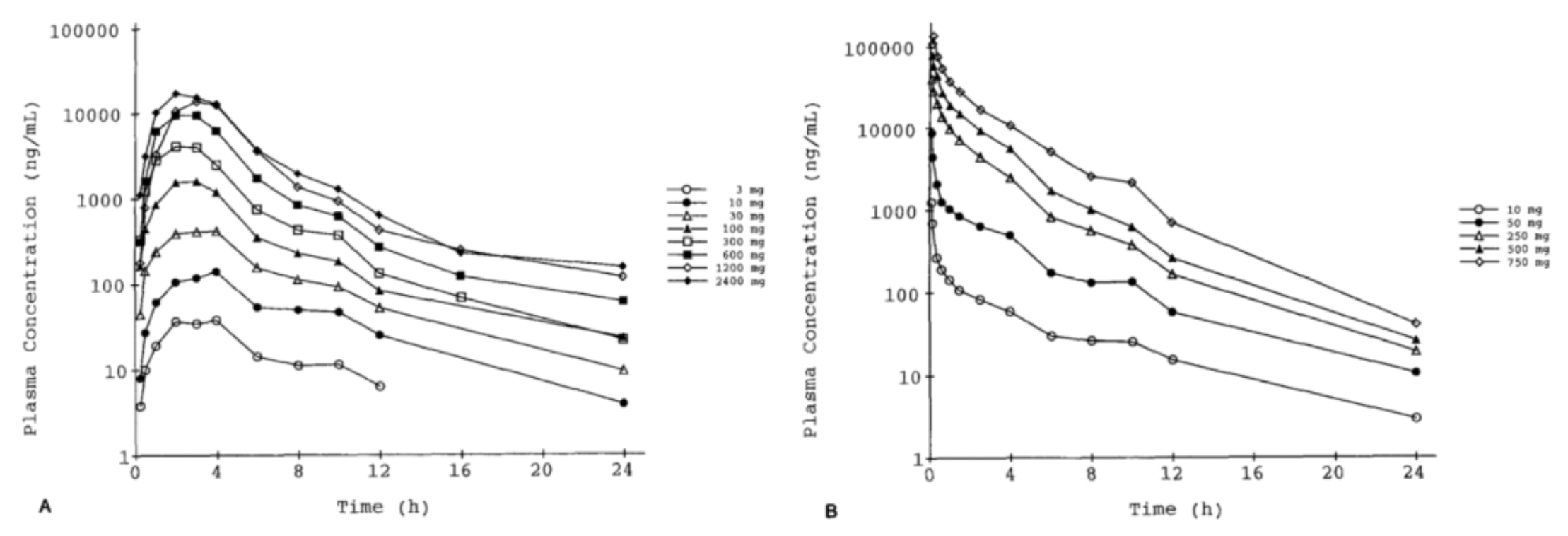
In 1996, the Roche team first published a pharmacokinetic and pharmacodynamic study of Bosentan in healthy humans in the journal "Clin Pharmacol Ther."
The results showed that the systemic plasma clearance and the volume of distribution for Bosentan decreased with an increase in dose, with the maximum values being approximately 6 L/h and 0.2 L/kg, respectively. The absolute bioavailability was 50% and seemed to decrease with doses exceeding 600 mg. The maximum increase in plasma ET-1 was 2-fold (oral) and 3-fold (intravenous). Bosentan reversed the vasoconstrictive effects of ET-1 measured in the skin microcirculation, with a downward trend in blood pressure (about 5 mm Hg) and an upward trend in pulse rate (about 5 beats/min). Oral administration of Bosentan was well-tolerated, whereas larger doses administered intravenously caused vomiting and local intolerance.
PIVLAZ® (Clazosentan) is a potent and selective endothelin ETA receptor antagonist developed by Idorsia Pharmaceuticals for the prevention of cerebral vasospasm, vasospasm-related cerebral infarction, and cerebral ischemic symptoms following aneurysmal subarachnoid hemorrhage (aSAH). Pivlaz® was successfully launched in Japan in April 2022 for the treatment of cerebral vasospasm. Sosei Heptares holds the marketing rights for Pivlaz® in Japan and the Asia-Pacific region, excluding China. (For further reading on endothelin receptors ETA/ETB: GPCR3D - Current pharmaceutical research on Endothelin receptors (ETA/ETB)).
It is reported that 40% to 70% of patients experience cerebral vasospasm within 4 to 14 days after an aneurysmal subarachnoid hemorrhage (aSAH). Among these patients, 17% to 40% will develop delayed ischemic neurological deficits, and about half of these individuals may suffer a cerebral infarction. Following acute cerebral infarction, levels of endothelin increase induced by oxyhemoglobin and released from red blood cells, causing cerebral vasospasm via the endothelin ETA receptor.
According to a clinical study report published by the Idorsia team in 2022, 221 patients received randomized treatments in each study, and 220 patients were included in the full analysis set for the primary analysis (109 in the Clazosentan group and 111 in the placebo group). Compared to placebo, a 150 mg intravenous infusion of Clazosentan significantly reduced the incidence of vasospasm-related morbidity and all-cause mortality after aSAH.

Aprocitentan is a novel oral dual endothelin receptor antagonist currently being developed by Idorsia Pharmaceuticals. It effectively inhibits the binding of ET-1 to ETA and ETB receptors. It is reported that the potential for drug-drug interactions with Aprocitentan is low, and its mechanism of action is well-suited for the pathophysiology of resistant hypertension.
In November 2022, the team from Idorsia partnered with Johnson & Johnson to publish data from a phase III clinical study on the use of aprocitentan for the treatment of resistant hypertension in The Lancet journal. The results indicated that in patients with resistant hypertension, aprocitentan was well-tolerated, demonstrated better blood pressure reduction than placebo at week 4, and its antihypertensive effects were sustained through to week 40.
Sparsentan (BMS-346567) was developed by the BMS research team in 2005 as the first oral active drug of its kind. Sparsentan is a hybrid structure combining the structural elements of the AT1R antagonist irbesartan and the ETAR antagonist biphenylsulfonamide. Sparsentan has a high affinity for both receptors, with an affinity of 9.3 nM for ETAR and 0.8 nM for AT1R.
In May 2023, the prestigious medical journal The Lancet published online the clinical study results of Travere Therapeutics regarding Sparsentan in patients with IgA nephropathy. The results indicate that compared to the single ATR antagonist irbesartan, the daily treatment with the dual-acting agent Sparsentan significantly reduces proteinuria in adult patients with IgA nephropathy.