The "Antidote" Specific to Biological Toxins: Antitoxin
Antitoxin is a specific antibody or serum containing this antibody that can neutralize toxins. By using toxoids for immunization, the body can produce corresponding antitoxins to prevent diseases.
Antibodies are glycoproteins produced by plasma cells after B cells are stimulated by antigens, which mainly exist in serum and other body fluids. They are important effector molecules mediating humoral immunity and can bind specifically with corresponding antigens to perform immune functions.
Essentially, antitoxins are the corresponding antibodies or immune serums containing these antibodies to bacterial toxins (usually referring to exotoxins). They can neutralize the toxic effects of corresponding exotoxins. The body can produce antitoxins against pathogens that cause disease by producing exotoxins, such as diphtheria, tetanus, and gas gangrene bacteria. After treatment with formaldehyde, exotoxins can lose their toxicity while maintaining their immunogenicity, becoming toxoids.
Antitoxin Competitive Landscape
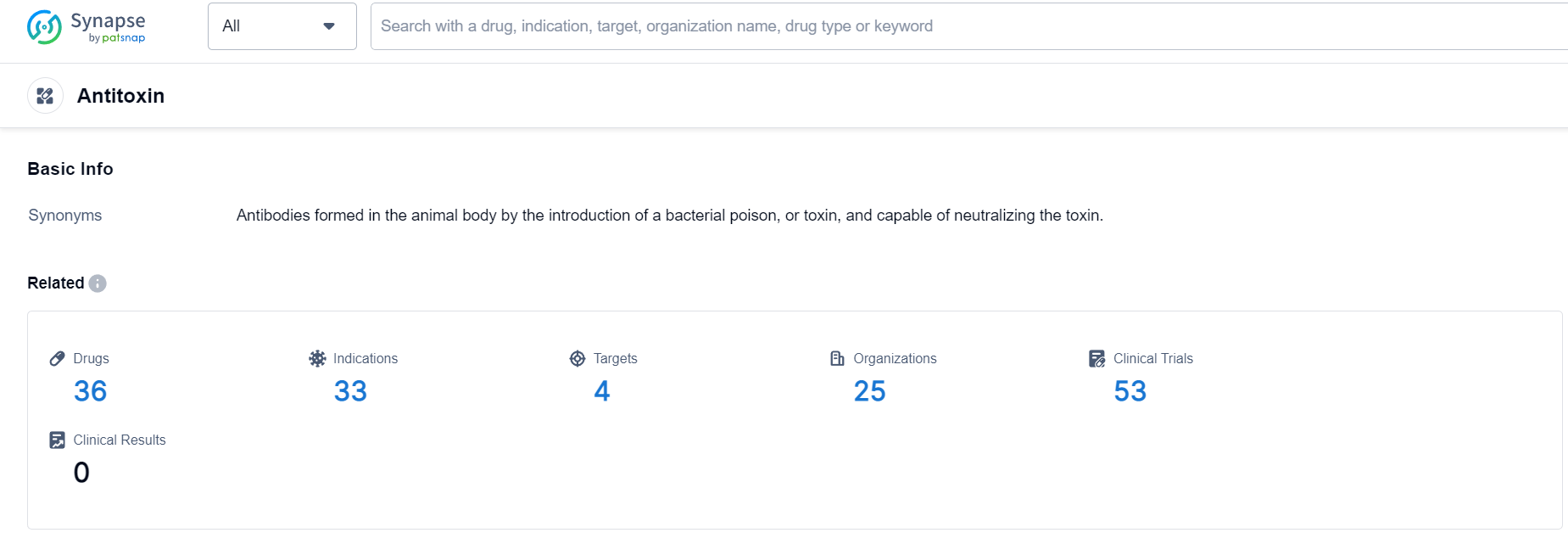
According to Patsnap Synapse, as of 24 Sep 2023, there are a total of 36 Antitoxin drugs worldwide, from 25 organizations, covering 4 targets, 33 indications, and conducting 53 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
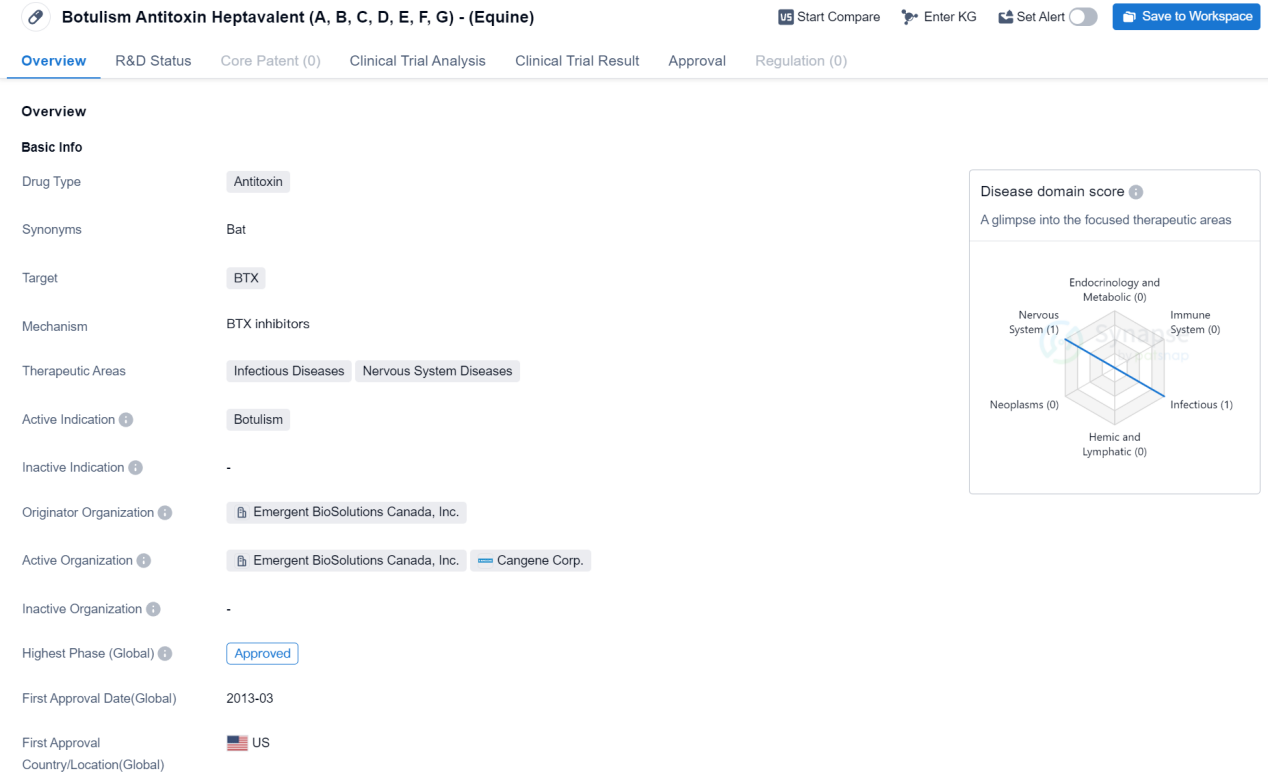
Approved Antitoxon Medicinal: Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G)
The drug Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G) - (Equine) is an antitoxin that targets BTX, which stands for botulinum toxin. It is primarily used for the treatment of botulism, a rare but serious illness caused by the toxin produced by the bacterium Clostridium botulinum. Botulism can lead to muscle weakness, paralysis, and potentially life-threatening complications.
The drug falls under the therapeutic areas of infectious diseases and nervous system diseases, as it specifically addresses the effects of botulinum toxin on the body. By neutralizing the toxin, the antitoxin helps to alleviate the symptoms and prevent further damage caused by botulism.
The drug received its first approval in the United States in March 2013. The originator organization of this drug is Emergent BioSolutions Canada, Inc. They are responsible for the development and production of the drug. The highest phase of development for this drug is approved, indicating that it has successfully completed clinical trials and has been granted regulatory approval for use.
In summary, Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G) - (Equine) is an antitoxin drug developed by Emergent BioSolutions Canada, Inc. It is used for the treatment of botulism, a serious illness caused by botulinum toxin. The drug has been approved and was first authorized for use in the United States in March 2013.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
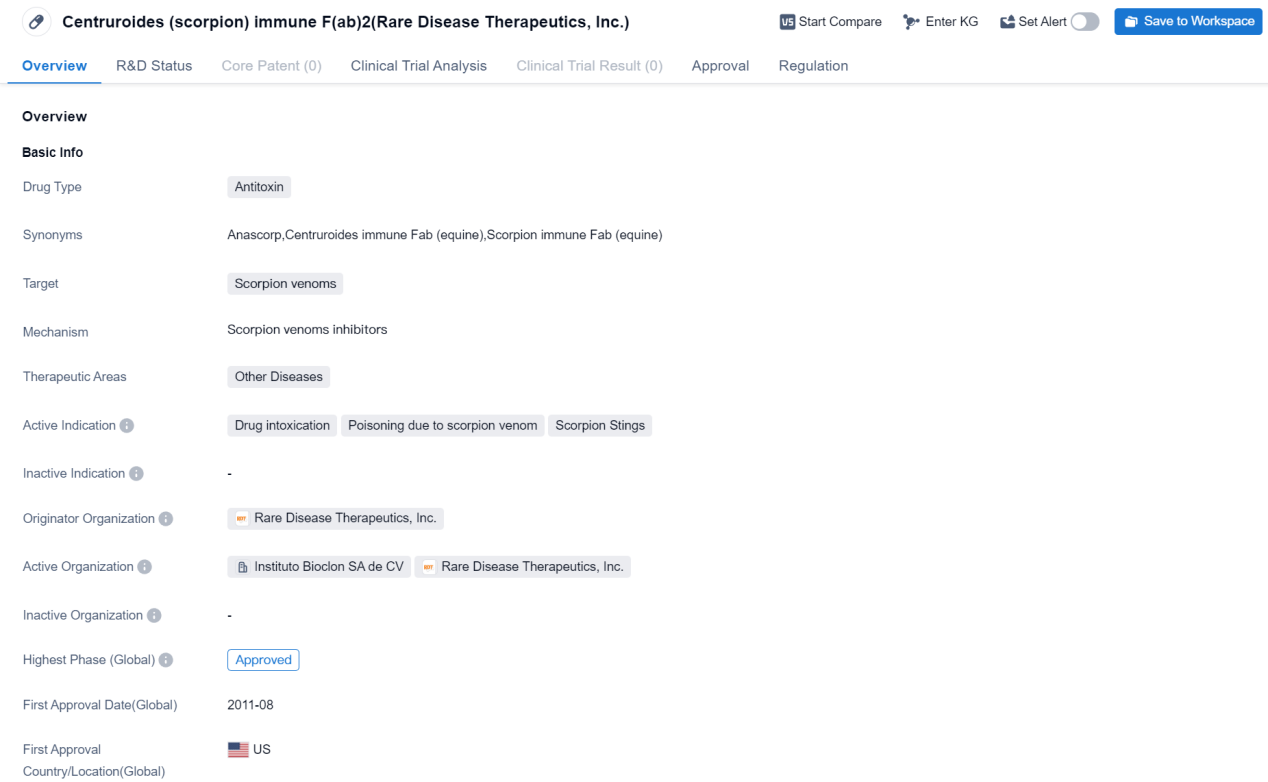
Approved Antitoxon Medicinal: Centruroides (scorpion) immune F(ab)2
Centruroides (scorpion) immune F(ab)2 is an antitoxin drug developed by Rare Disease Therapeutics, Inc. It falls under the therapeutic area of Other Diseases and is specifically designed to target scorpion venoms. The drug is primarily indicated for the treatment of drug intoxication, poisoning due to scorpion venom, and scorpion stings.
Centruroides (scorpion) immune F(ab)2 received its first approval in the United States in August 2011. It is classified as an orphan drug, indicating that it is intended to treat rare diseases or conditions.
As an antitoxin, Centruroides (scorpion) immune F(ab)2 works by neutralizing the toxic effects of scorpion venoms. Scorpion stings can cause severe symptoms, including pain, swelling, and potentially life-threatening complications. This antitoxin aims to alleviate these symptoms and improve patient outcomes.
The drug has successfully completed its highest phase of development and has been approved for use. The approval of Centruroides (scorpion) immune F(ab)2 highlights its potential as a valuable treatment option for individuals affected by scorpion venom-related conditions.
Since its approval, Centruroides (scorpion) immune F(ab)2 has been available in the United States to address the specific medical needs of patients suffering from scorpion venom intoxication, poisoning, and stings. The drug's approval as an orphan drug reflects the recognition of the limited treatment options available for these rare conditions and the importance of providing targeted therapies.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, Centruroides (scorpion) immune F(ab)2 is an antitoxin drug developed by Rare Disease Therapeutics, Inc. It is indicated for the treatment of drug intoxication, poisoning due to scorpion venom, and scorpion stings. The drug received its first approval in the United States in 2011 and is classified as an orphan drug. Its approval signifies its successful completion of the highest phase of development and highlights its potential as a valuable treatment option for individuals affected by scorpion venom-related conditions.