Ionis and Biogen Release Key Findings from Phase 1/2 Trial of Experimental ALS Drug
Ionis Pharmaceuticals, Inc. and Biogen Inc. have decided to discontinue the development of BIIB105 (ION541), an experimental antisense oligonucleotide aimed at treating amyotrophic lateral sclerosis (ALS), following the initial outcomes from the Phase 1/2 ALSpire trial. BIIB105 was engineered to lower the levels of the ataxin-2 (ATXN2) protein and showed a significant decrease in cerebrospinal fluid ATXN2 protein in the study.
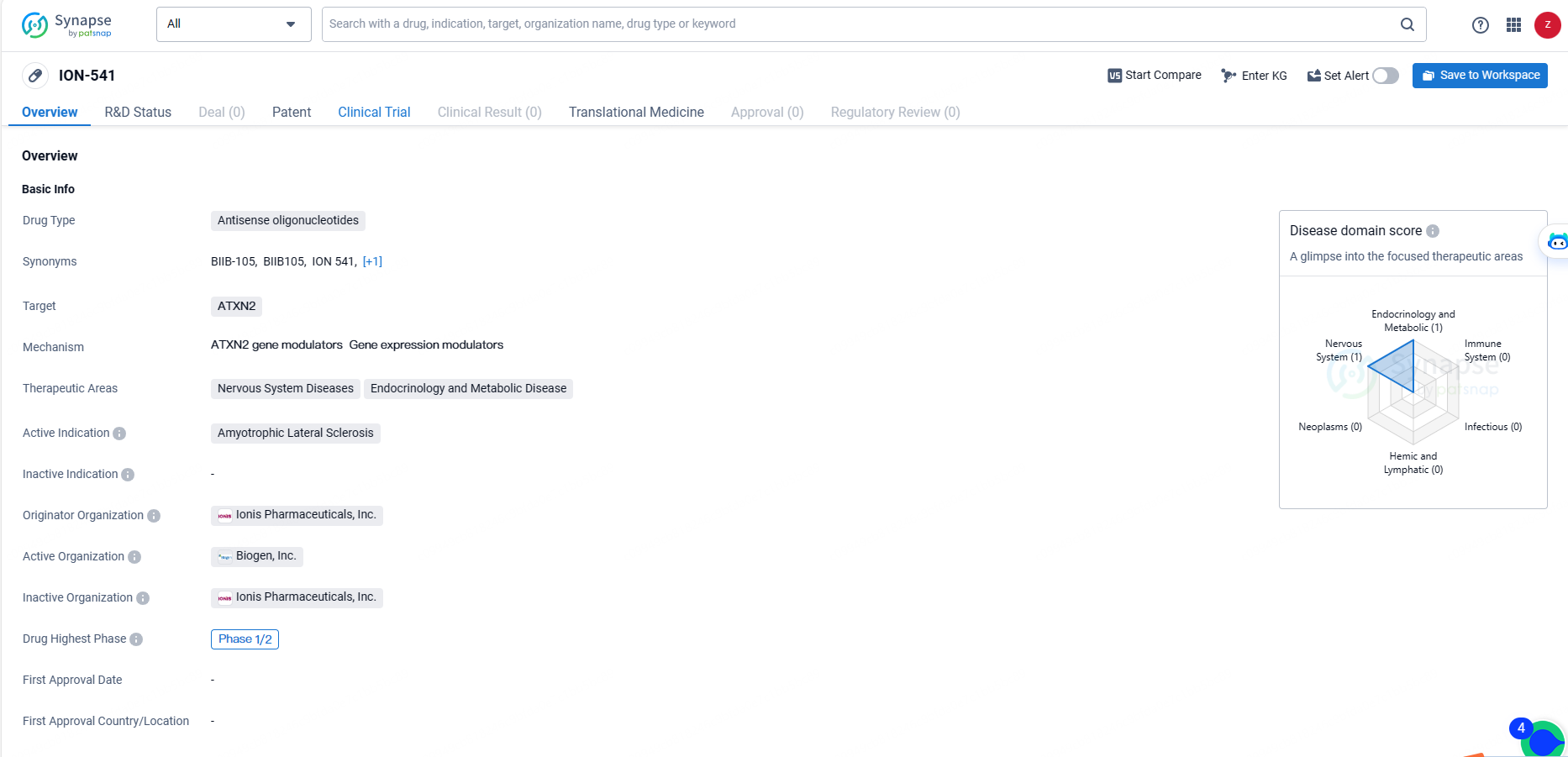
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Over a 6-month period of being compared to a placebo, BIIB105 treatment did not show a decrease in plasma neurofilament light chain levels, a biomarker for neurodegeneration and neuronal damage. Furthermore, BIIB105 had no significant effect on functional, respiratory, or strength-related clinical outcomes.
"Although BIIB105 lowered ATXN2 protein levels, it did not lead to a reduction in neurofilament, indicating that the drug did not decelerate the disease progression," stated Stephanie Fradette, Pharm.D., Head of Biogen's Neuromuscular Development Unit. "We are immensely grateful for the study participants' contributions and remain committed to advancing therapies that can significantly alter the course of ALS."
"We deeply appreciate the efforts of the ALS patients and investigators who took part in this study, which was critical for advancing our scientific knowledge," remarked Frank Bennett, Ph.D., Executive Vice President and Chief Scientific Officer of Ionis. "Ionis remains dedicated to the ALS community and is progressing with our Phase 3 ulefnersen program for individuals with the genetic subtype known as FUS-ALS."
The long-term biomarker and efficacy data from the open-label extension mirrored those of the 6-month placebo-controlled period, showing sustained reductions in ATXN2 without affecting NfL or clinical outcomes over more than 40 weeks of follow-up. No subgroup, including participants with a Poly-CAG expansion in the ATXN2 gene, demonstrated any signs of benefit.
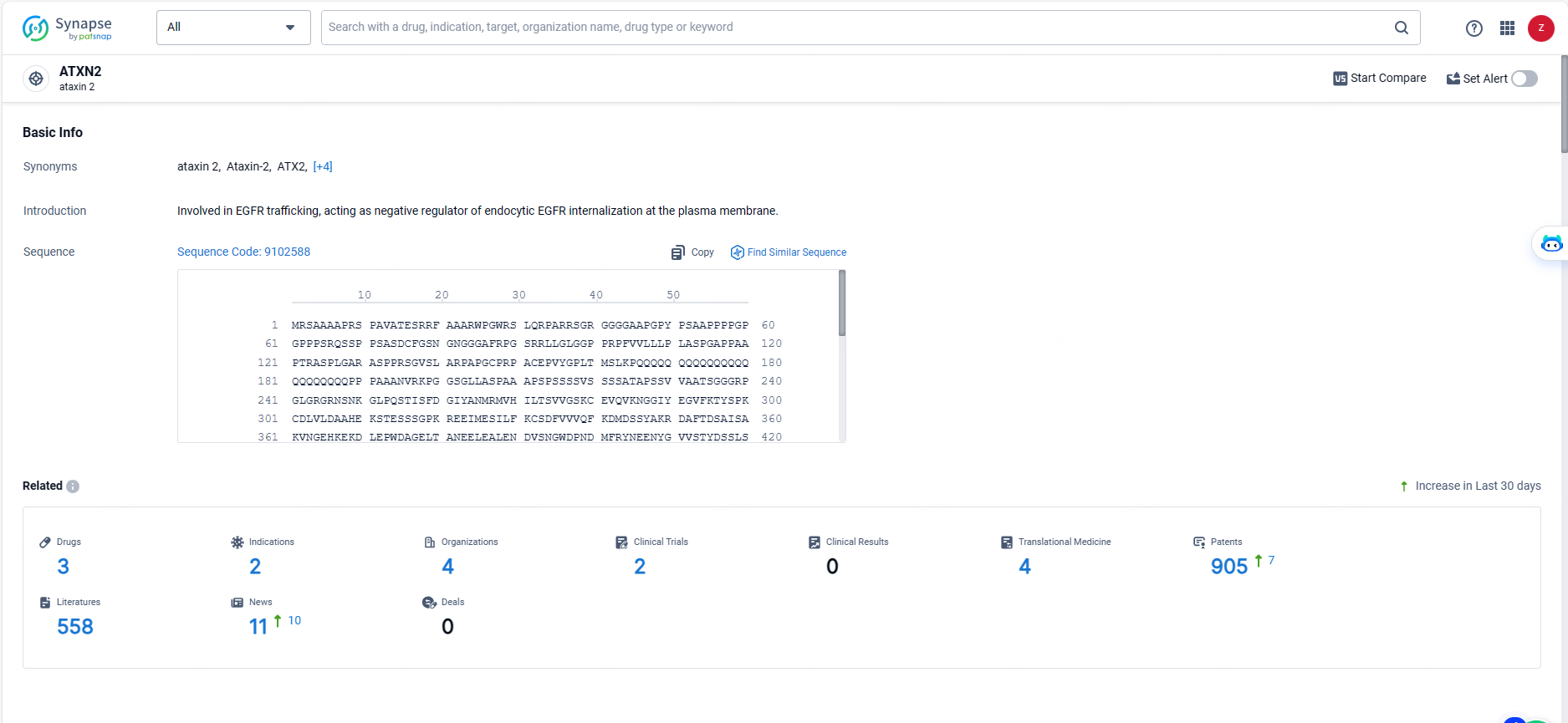
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 20, 2024, there are 3 investigational drugs for the ATXN2 target, including 2 indications, 4 R&D institutions involved, with related clinical trials reaching 2, and as many as 905 patents.
ION-541 is an antisense oligonucleotide drug targeting ATXN2, with a primary focus on treating Amyotrophic Lateral Sclerosis. Its development is at the Phase 1/2 stage, and it holds potential for addressing not only ALS but also other nervous system and metabolic diseases. ION-541 represents the company's commitment to advancing novel therapies in the field of biomedicine.