Nature: Dual-active molecules targeting GLP-1R and NMDA receptors exhibit superior anti-obesity effects
On May 15th, the journal Nature published a research paper titled "GLP-1-directed NMDA receptor antagonism for obesity treatment" by the Novo Nordisk team and the team led by Christoffer Clemmensen at the University of Copenhagen. The article discusses the development of a dual-functional molecule named GLP-1–MK-801 and its preclinical progress in preventing metabolic diseases.
This molecule is designed to integrate NMDA receptor antagonism with glucagon-like peptide-1 (GLP-1) receptor agonism, successfully reversing obesity, hyperglycemia, and dyslipidemia in rodent models. By directing the delivery of the NMDA receptor antagonist MK-801 via GLP-1 pathways, the researchers were able to influence neuroplasticity in the hypothalamus and brainstem. Additionally, targeting MK-801 to brain regions expressing GLP-1 receptors avoided the adverse physiological and behavioral effects associated with standalone MK-801 treatment.

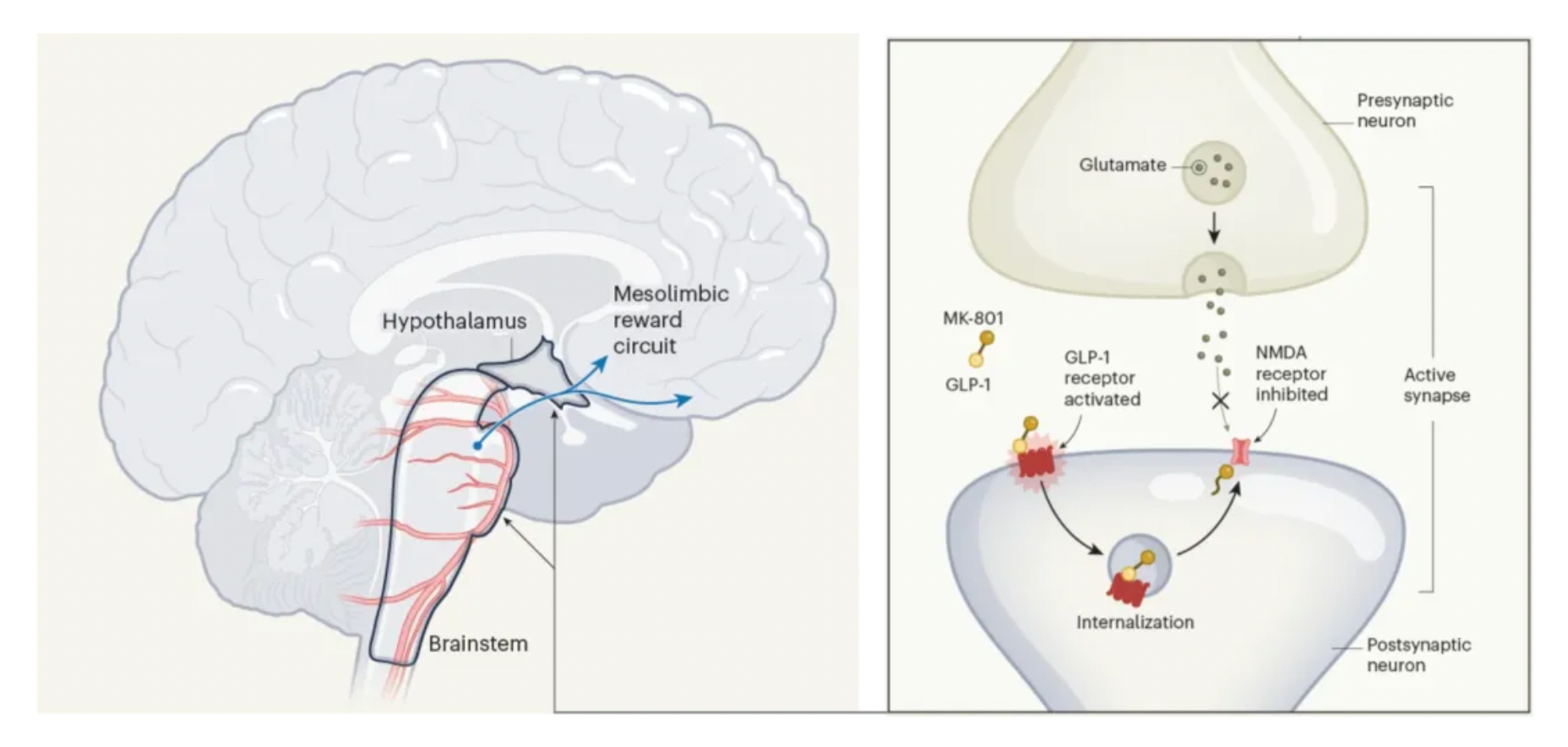
The drug is composed of a GLP-1 receptor agonist combined with an inhibitor (antagonist) of another receptor extensively present in the brain—the NMDA receptor. NMDA receptors bind to the neurotransmitter molecule glutamate and are crucial for regulating synaptic plasticity, a process that allows communication between neurons to adapt by strengthening or weakening connection strength based on activity patterns.
The signaling pathways involving NMDA receptors and glutamate have been associated with obesity in human genomic studies, further reinforcing the rationale behind this pharmacological strategy. Although targeting NMDA receptors to reduce weight is not a novel concept, previous attempts have failed due to the side effects associated with systemic inhibition of NMDA receptors, such as hyperthermia and hyperactivity.
The author's dual-acting compound addresses this issue: the NMDA receptor antagonist (MK-801) is only activated when the drug binds to and is internalized by the GLP-1 receptor. Subsequently, MK-801 can attenuate neuronal excitability, and due to its conjunction with the GLP-1 receptor agonist, this effect is confined to neurons expressing the GLP-1 receptor.
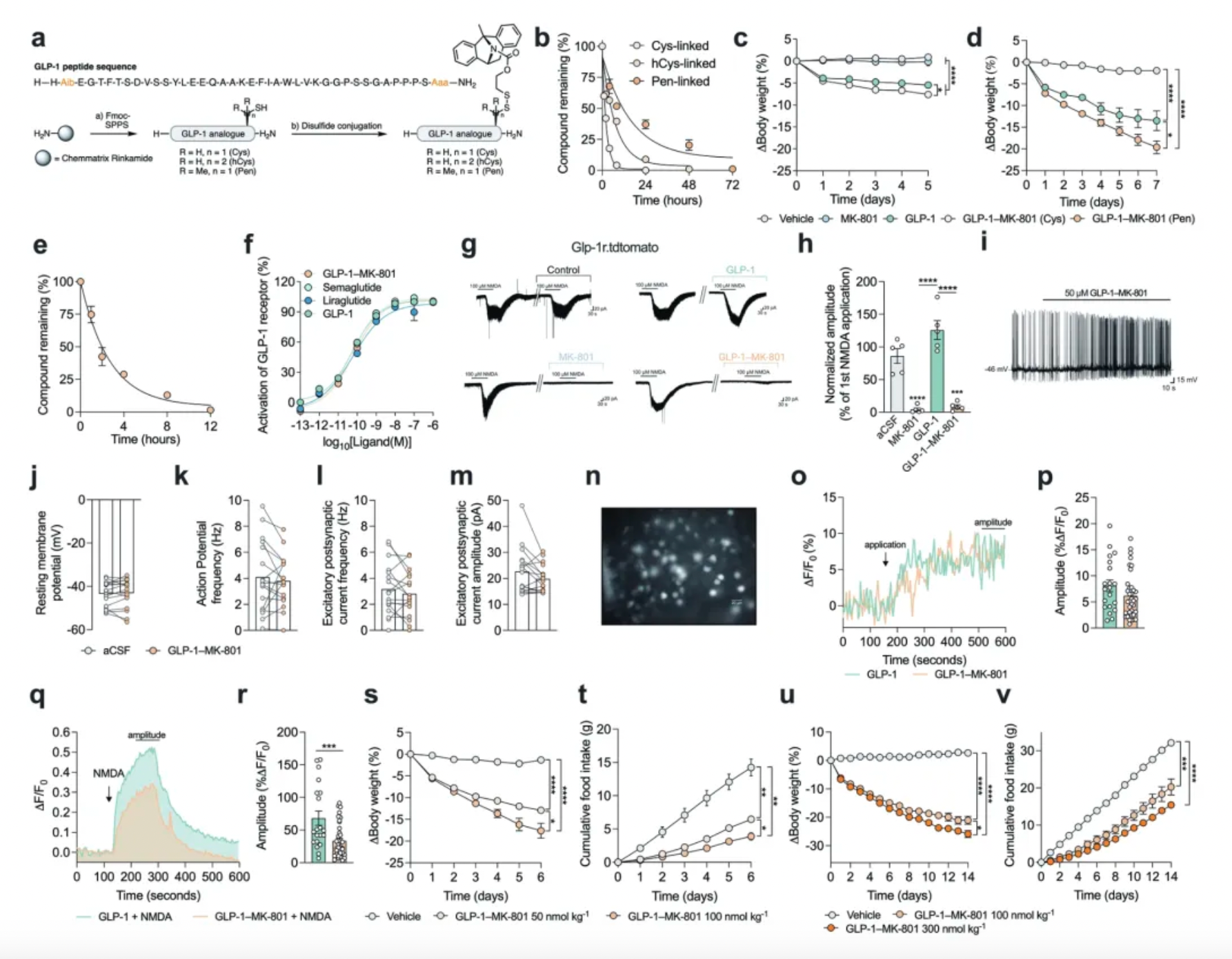
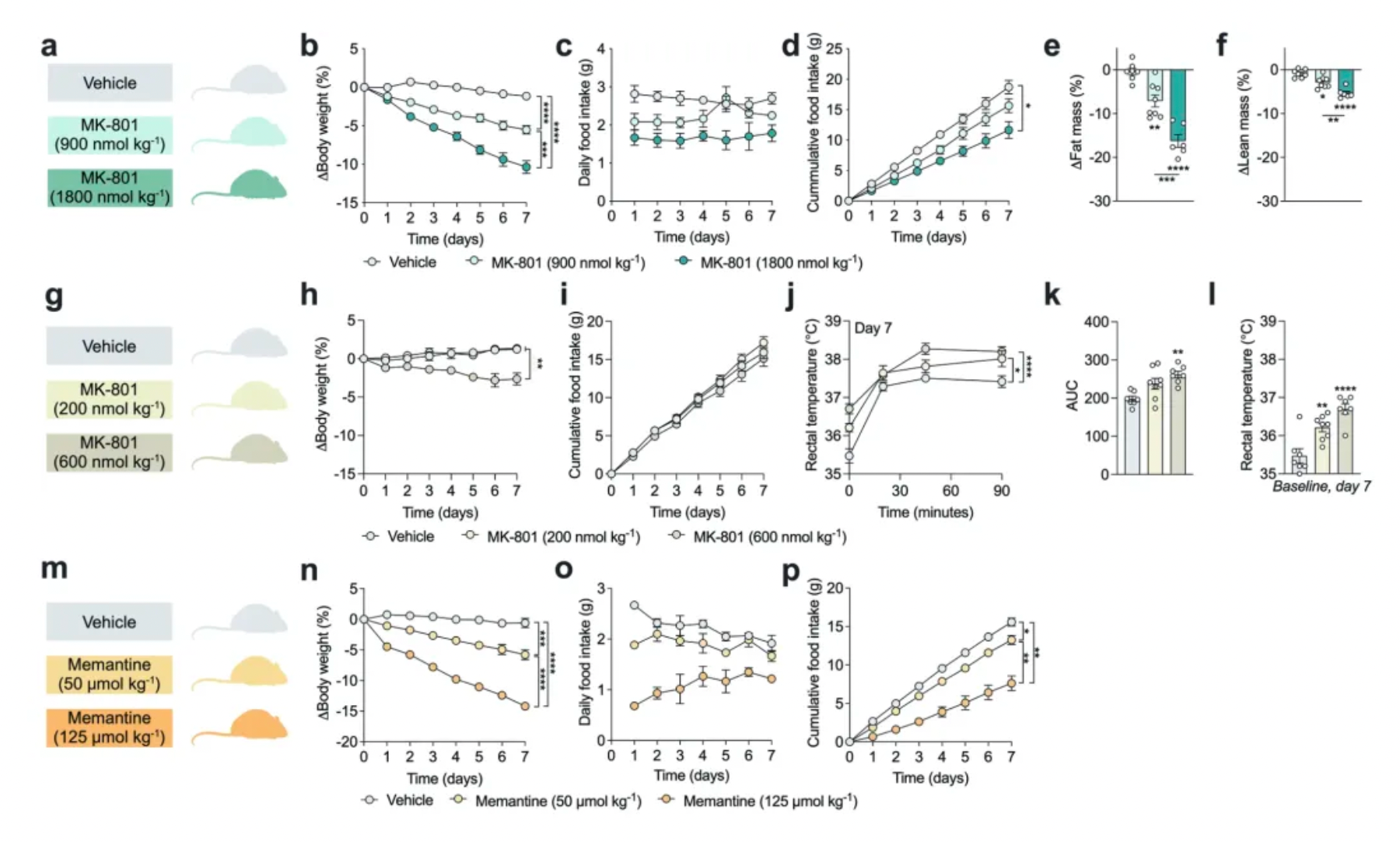
Research has found that, compared to standalone GLP-1 analogs and other GLP-1 receptor agonists (including Semaglutide), GLP-1-MK-801 exhibits similar in vivo pharmacokinetics in rats and mice but is more effective in reducing body weight.
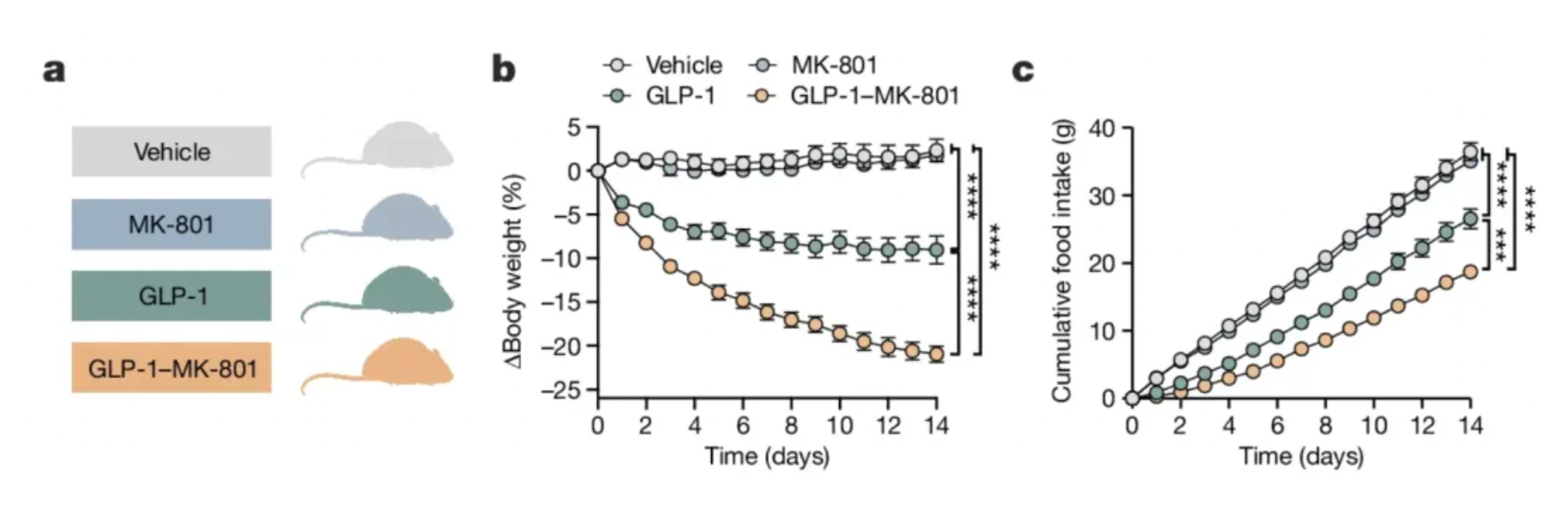
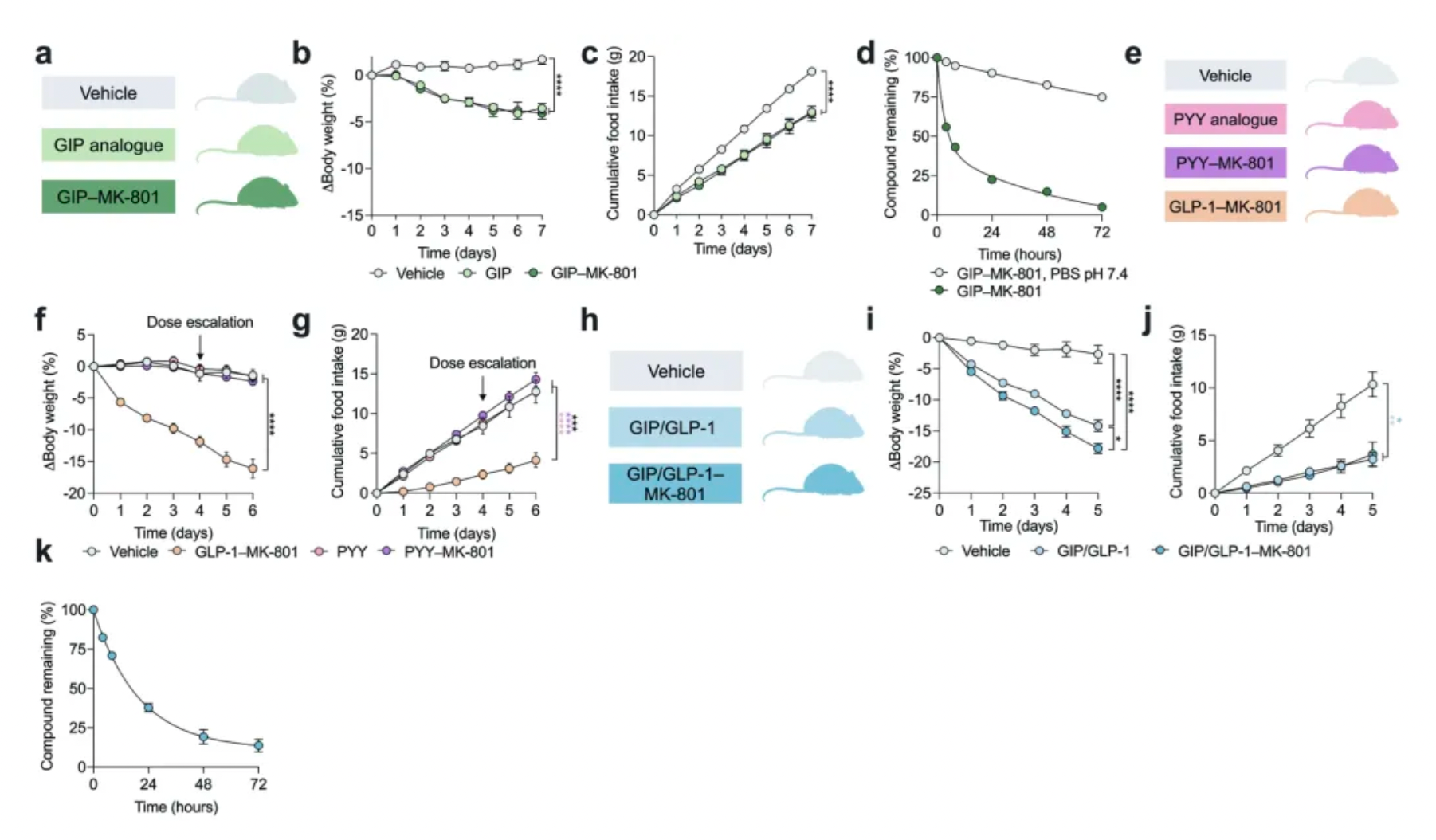
Focusing the inhibition of NMDA receptors specifically on neurons expressing the GLP-1 receptor is essential for achieving this effect. However, conjugating MK-801 with other gut peptides thought to suppress food intake (such as GIP, PYY, etc.) does not prove to be more effective in reducing body weight than using MK-801 or GLP-1 analogs alone.
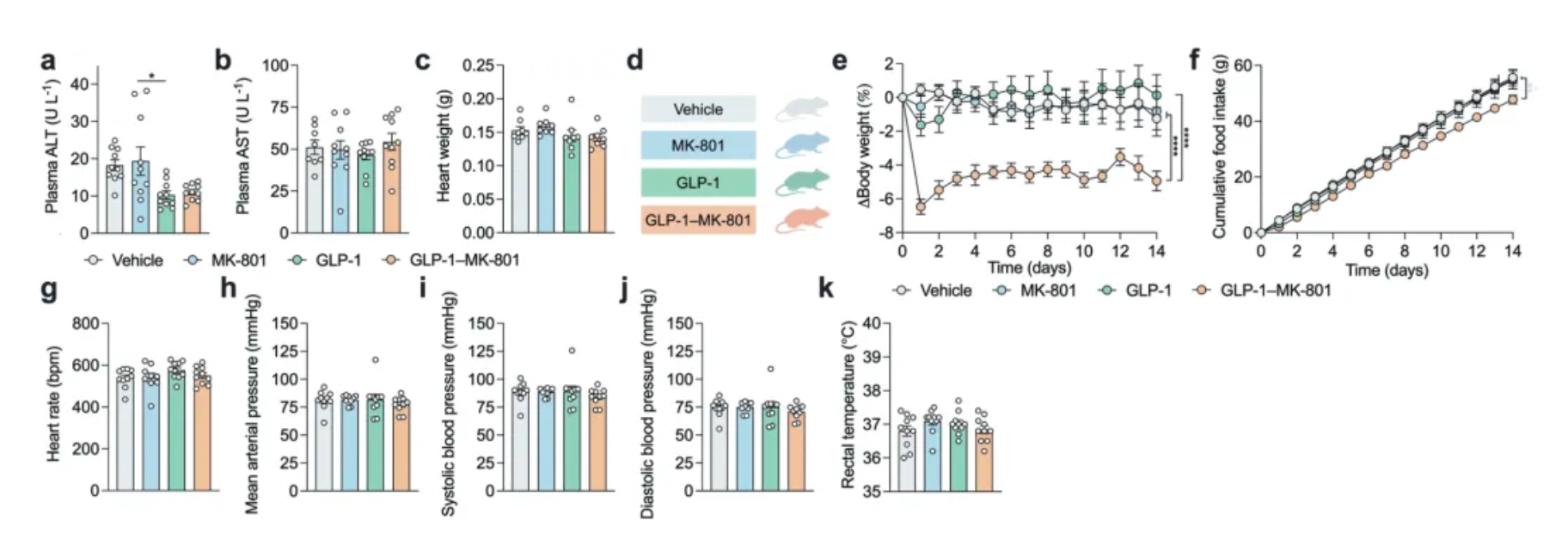
In addition to demonstrating improvements in cardiometabolic health without signs of liver damage, animals treated with GLP-1-MK-801 exhibited relatively mild common side effects associated with GLP-1 agonists (nausea) and NMDA receptor antagonists (hyperthermia and hyperactivity).
Interestingly, animals treated with GLP-1-MK-801 lost more weight than those whose food intake was similarly restricted but were on a calorie-limited diet. This suggests that the drug’s influence on energy balance extends beyond merely reducing food intake. By studying the animals in metabolic cages that measure metabolic rate, it was further explored that, despite the GLP-1-MK-801-treated mice being significantly smaller in size and consuming less food than the obese control group, they burned the same amount of calories. Therefore, the drug may overcome a challenge of diet and exercise, where the brain compensates for weight loss by lowering metabolic rate, making it difficult to maintain lower weight long-term.
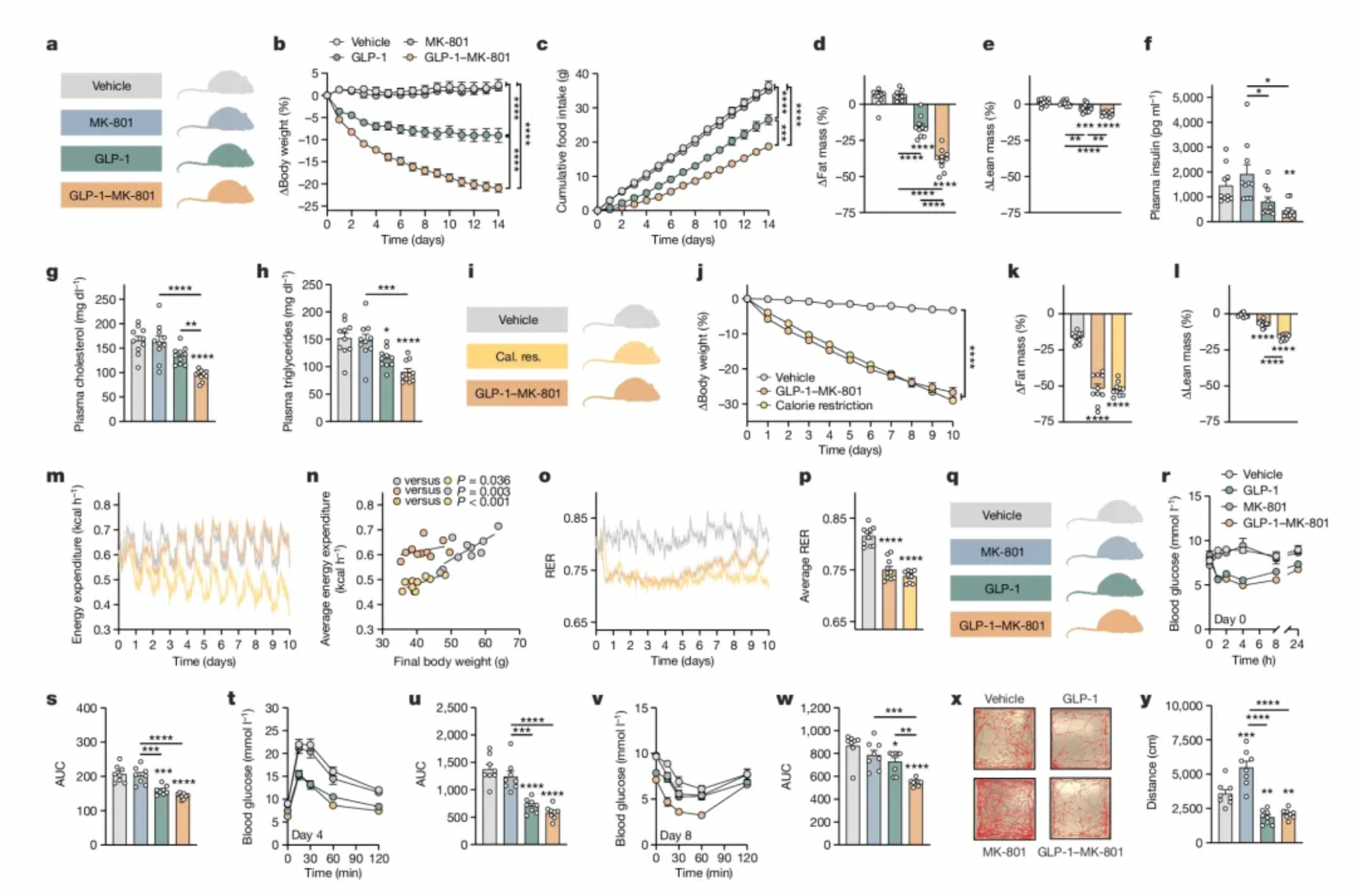
Researchers have also found that GLP-1-MK-801 alters the gene expression in signaling pathways associated with both GLP-1 and MK-801, suggesting a coordinated interaction between these two receptor systems. In some respects, the mechanism of GLP-1-MK-801 is similar to other therapies targeting GLP-1 receptors, in that it activates neural circuits involved in feeding, appetite, and reward. However, compared to semaglutide, it more intensely activates brain regions that regulate reward, such as the limbic system.
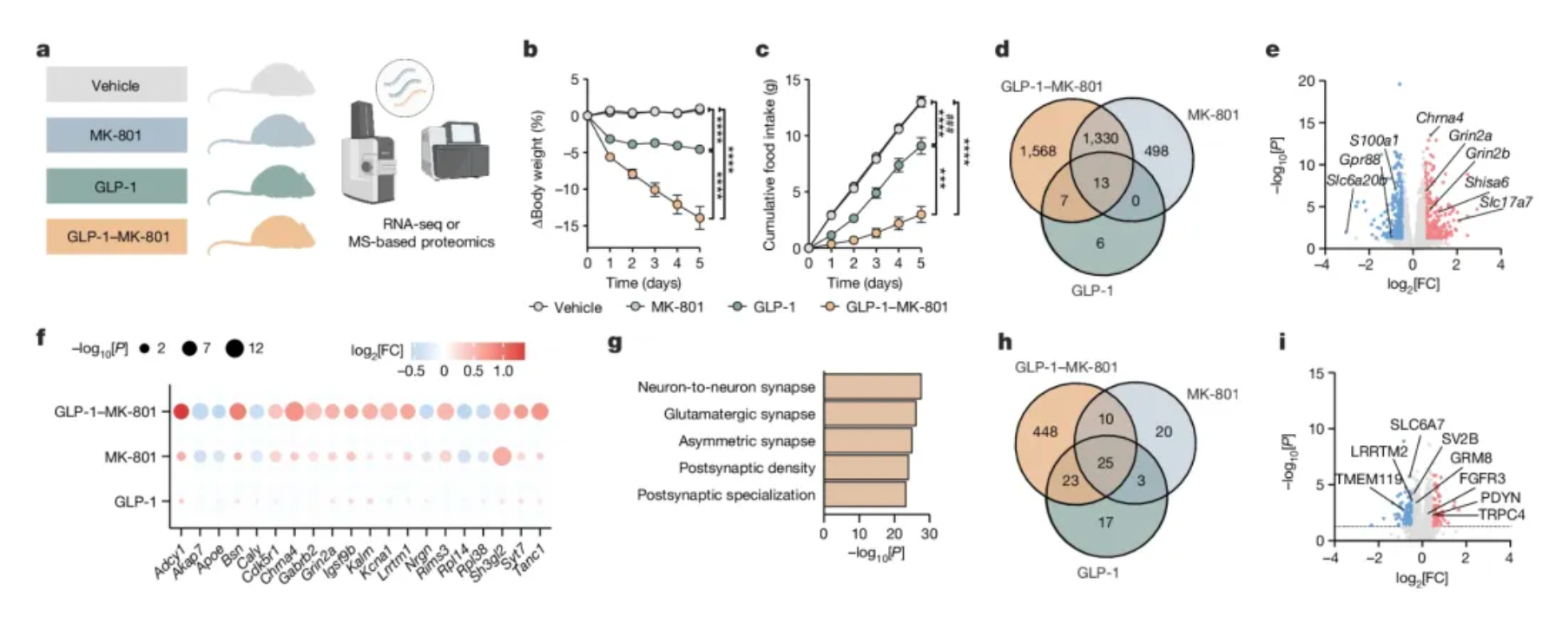
A single-dose, direct brain injection of GLP-1-MK-801 has a more lasting effect on reducing food intake compared to an injection of semaglutide alone. This suggests that the impact on synaptic plasticity might be long-term.
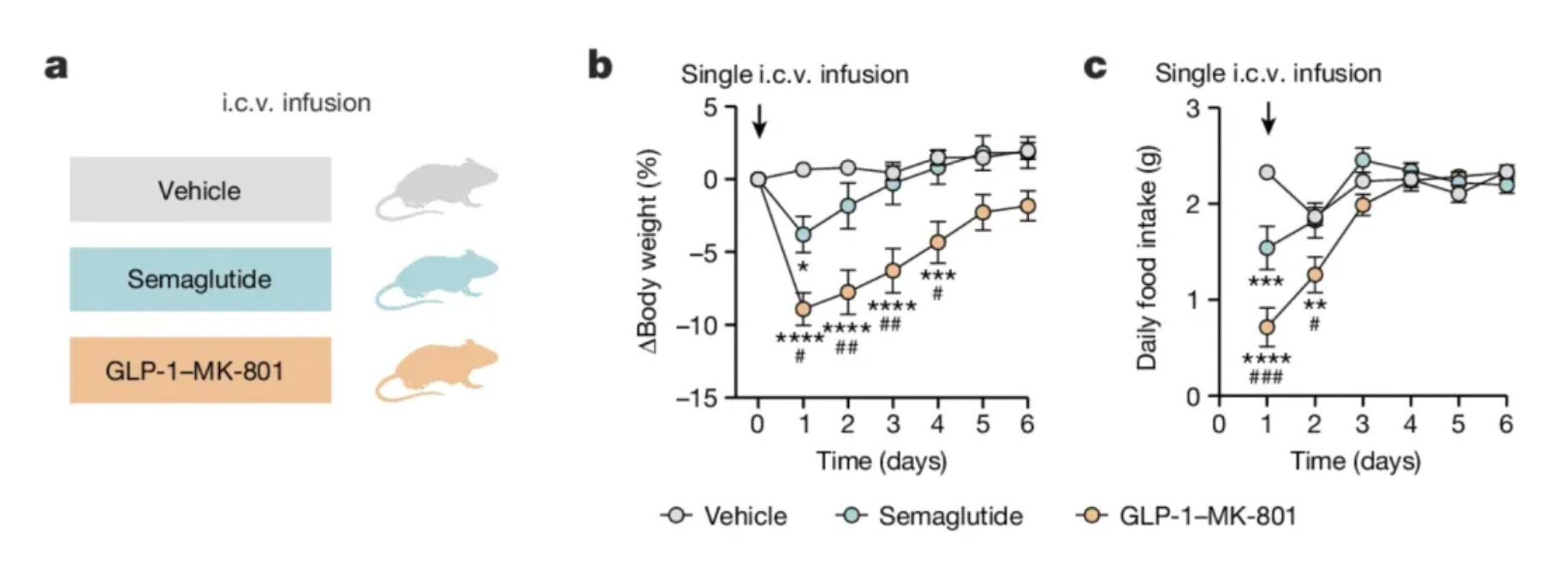
Another major drawback of drug-mediated weight loss is that individuals tend to regain weight once they stop taking the medication. This implies that, similar to other cardiovascular and metabolic treatments, people cannot discontinue the medication if they wish to maintain their weight loss. Given the high cost of current GLP-1 receptor agonists, some individuals might find it unaffordable to sustain long-term obesity treatment. If the duotherapeutic molecule in this study alters neural plasticity, it could potentially be more economical than current anti-obesity drugs that require regular dosing. However, Peterson et al. only administered GLP-1-MK-801 to animals for up to two weeks, so its long-term efficacy and safety require further validation.
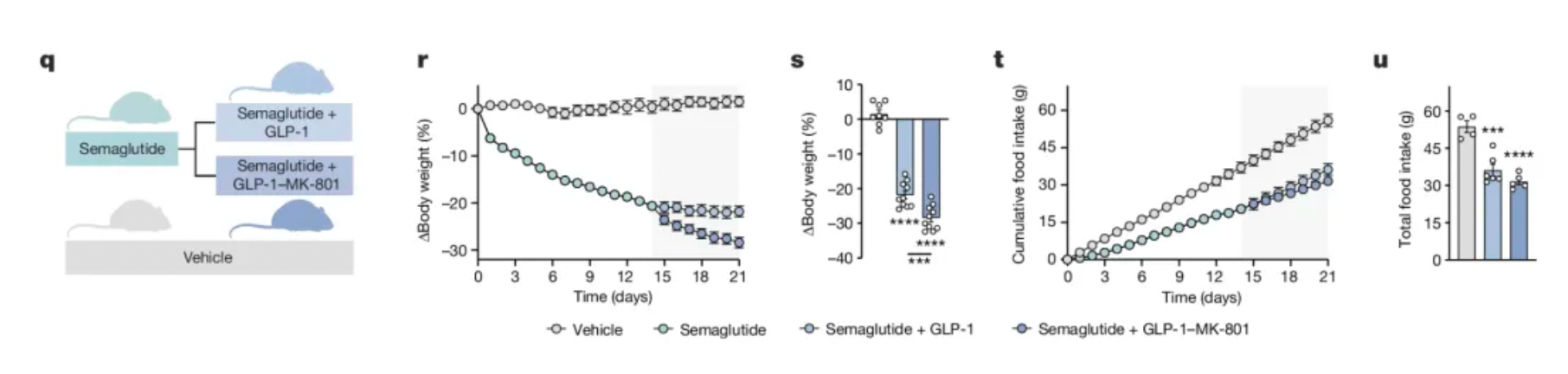
Finally, an important issue with GLP-1 receptor agonists is their potential to cause loss of lean body mass (fat-free mass). Unfortunately, GLP-1-MK-801 also induced significant lean body mass loss, but a group of food-restricted animals (restricted to achieve the same weight loss as the treated group) lost even more lean body mass. Therefore, GLP-1-MK-801 treatment might offer a slight advantage in preserving lean body mass compared to simple caloric restriction.
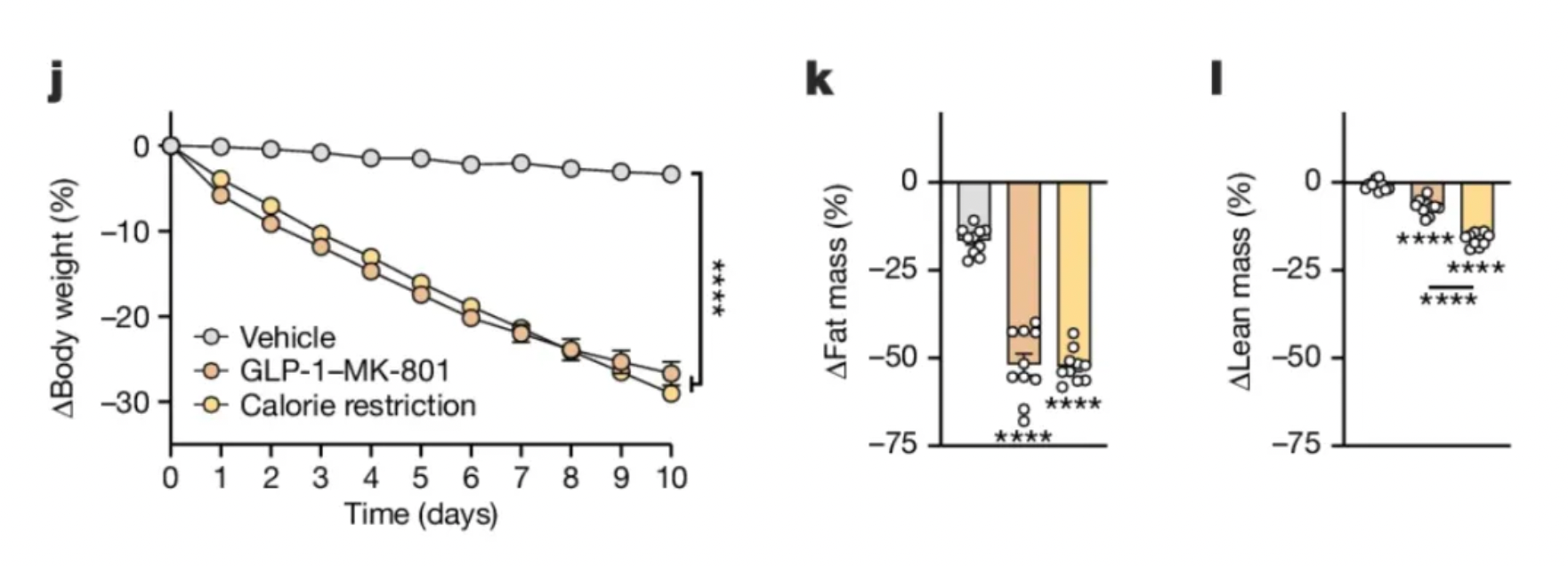
Although these data demonstrate the potential for targeted remodeling of neurons expressing the GLP-1 receptor to treat obesity, these preclinical findings must be evaluated in humans. Determining an optimal dosing regimen that maximizes fat loss, preserves muscle mass, and limits costs is the crucial next step. Given that GLP-1-MK-801 still leads to lean mass loss and food aversion (rather than just satiety), the drug appears to have some undesirable aspects. This innovative pharmacological strategy, along with the drug's ability to modulate synaptic plasticity, represents a crucial development in the efforts towards pharmacological interventions against obesity.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!