Is Deucravacitinib approved by the FDA?
Deucravacitinib, marketed under the brand name Sotyktu, was approved by the FDA on September 9, 2022, for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. It belongs to the drug class of multikinase inhibitors and is available in an oral tablet form with a dosage of 6 mg.
What is Deucravacitinib?
Deucravacitinib works by inhibiting multiple kinase pathways, thereby reducing inflammation and the overproduction of skin cells associated with plaque psoriasis. This medication can help manage the symptoms and improve the quality of life for those affected by this chronic skin condition.
Dosage and Administration:
- Recommended Dose: The usual adult dose for plaque psoriasis is 6 mg taken orally once a day, with or without food.
- Administration: The tablet should be swallowed whole without crushing, chewing, or breaking it.
- Monitoring: Patients will need medical tests before starting and while taking deucravacitinib to monitor for any potential side effects or complications.
Side Effects:
Common side effects of deucravacitinib may include cold symptoms (such as stuffy nose, sneezing, and sore throat), cold sores, sores on the gums, tongue or mouth, folliculitis, and acne. Serious side effects can include signs of infection, unexplained muscle pain or weakness, and symptoms of kidney failure. Patients are advised to seek emergency medical help if they experience any signs of an allergic reaction or serious side effects.
Warnings:
- Infections: Serious infections may occur during treatment. Patients should be vigilant for signs of infection and contact their doctor immediately if symptoms such as fever, chills, muscle pain, or skin sores develop.
- Muscle Tissue Breakdown: Deucravacitinib can cause muscle tissue breakdown, potentially leading to kidney failure. Unexplained muscle pain, tenderness, or weakness should be reported to a doctor immediately.
- Vaccinations: Patients should avoid receiving live vaccines while taking deucravacitinib due to potential interactions.
Precautions:
Before starting deucravacitinib, patients should inform their healthcare provider if they have a history of liver or kidney disease, hepatitis B or C, high triglycerides, cancer, tuberculosis, or if they are being treated for an infection. Patients should also inform their doctor if they are pregnant, planning to become pregnant, or breastfeeding.
Conclusion:
Deucravacitinib (Sotyktu) is an FDA-approved treatment for moderate to severe plaque psoriasis, offering a new therapeutic option for adults with this condition. Its approval on September 9, 2022, marks an important milestone in the management of plaque psoriasis, providing patients with an effective oral medication to control their symptoms and improve their quality of life. Patients should follow their healthcare provider's instructions carefully and be aware of the potential side effects and precautions associated with this medication.
How to obtain the latest development progress of all drugs?
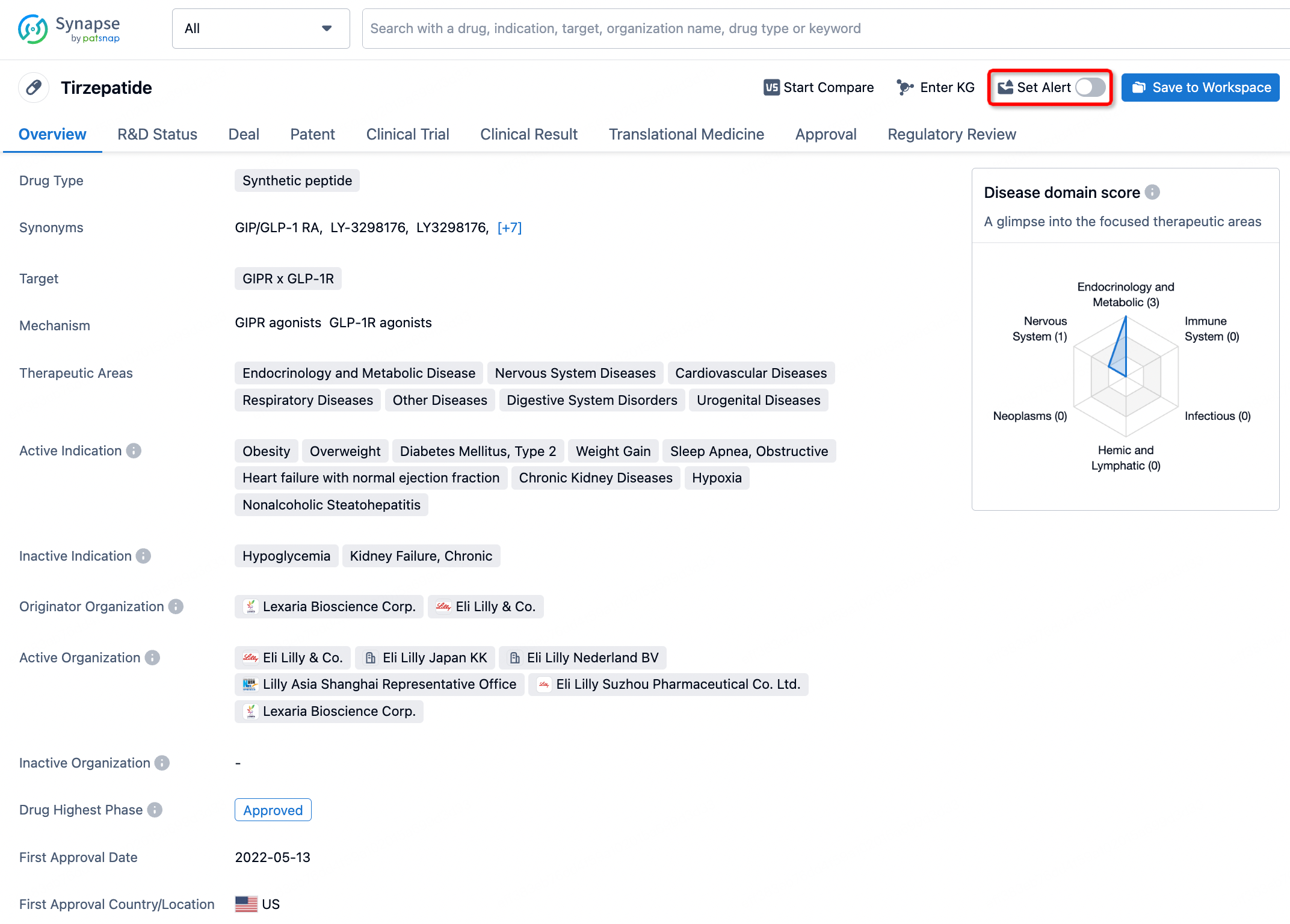
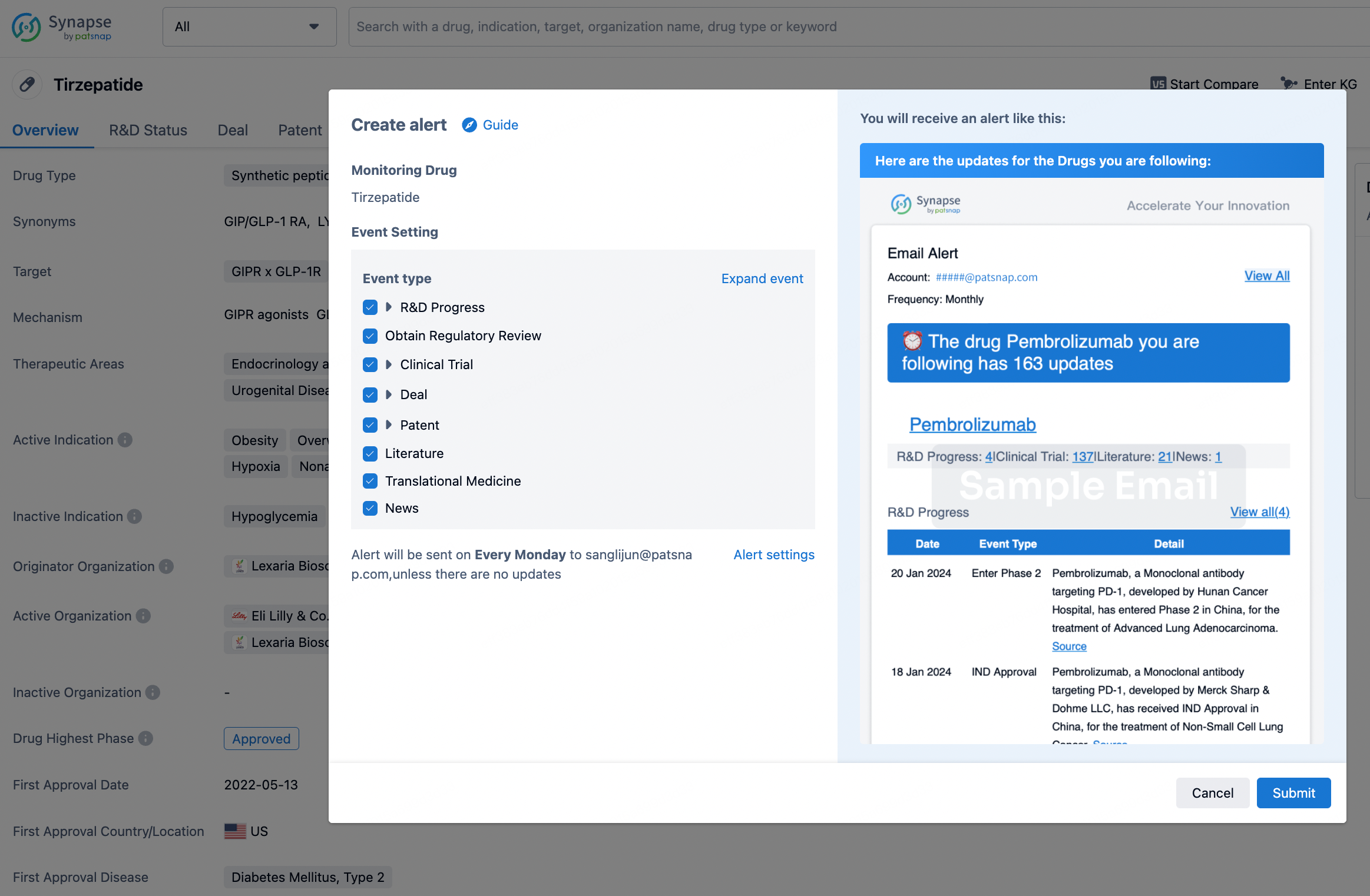
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!