Is Difelikefalin approved by the FDA?
Yes, Difelikefalin is FDA approved. The U.S. Food and Drug Administration (FDA) approved Difelikefalin on August 23, 2021, for the treatment of moderate-to-severe pruritus (itching) associated with chronic kidney disease in adults undergoing hemodialysis (HD).
Uses and Benefits
Difelikefalin is specifically used in adults who are on dialysis to manage itching associated with chronic kidney disease. This condition, known as pruritus, can significantly impact the quality of life for dialysis patients. Difelikefalin works as a peripheral opioid receptor agonist, helping to alleviate the discomfort caused by severe itching.
Administration and Dosage
Difelikefalin is administered as follows:
- Dosage Form: Intravenous solution (50 mcg/mL)
- Usual Adult Dose for Pruritus: 0.5 mcg/kg IV at the end of each hemodialysis treatment.
A healthcare provider administers Difelikefalin by IV bolus injection into the venous line of the dialysis circuit at the end of each HD treatment. The dose is determined by the patient's target dry body weight.
Side Effects
Common side effects of Difelikefalin may include:
- High blood levels of potassium (hyperkalemia)
- Diarrhea
- Nausea
- Headache
- Drowsiness
- Confusion
- Changes in mental state
- Dizziness
- Falls
- Loss of coordination
Serious side effects that require immediate medical attention include signs of an allergic reaction such as hives, difficulty breathing, and swelling of the face, lips, tongue, or throat.
Patients should report any side effects to their doctor and can also report them to the FDA at 1-800-FDA-1088.
Precautions
Before starting treatment with Difelikefalin, patients should consider the following precautions:
- Medical Conditions: Inform your doctor if you have trouble with standing, walking, or daily activities, changes in mental state, if you are on peritoneal dialysis, or if you have severe liver disease.
- Pregnancy and Breastfeeding: Tell your doctor if you are pregnant or breastfeeding.
Conclusion
Difelikefalin provides a significant treatment option for adults undergoing hemodialysis who suffer from moderate to severe pruritus associated with chronic kidney disease. It is crucial to follow the prescribed administration guidelines, take necessary precautions, and consult healthcare providers for personalized medical advice and treatment plans.
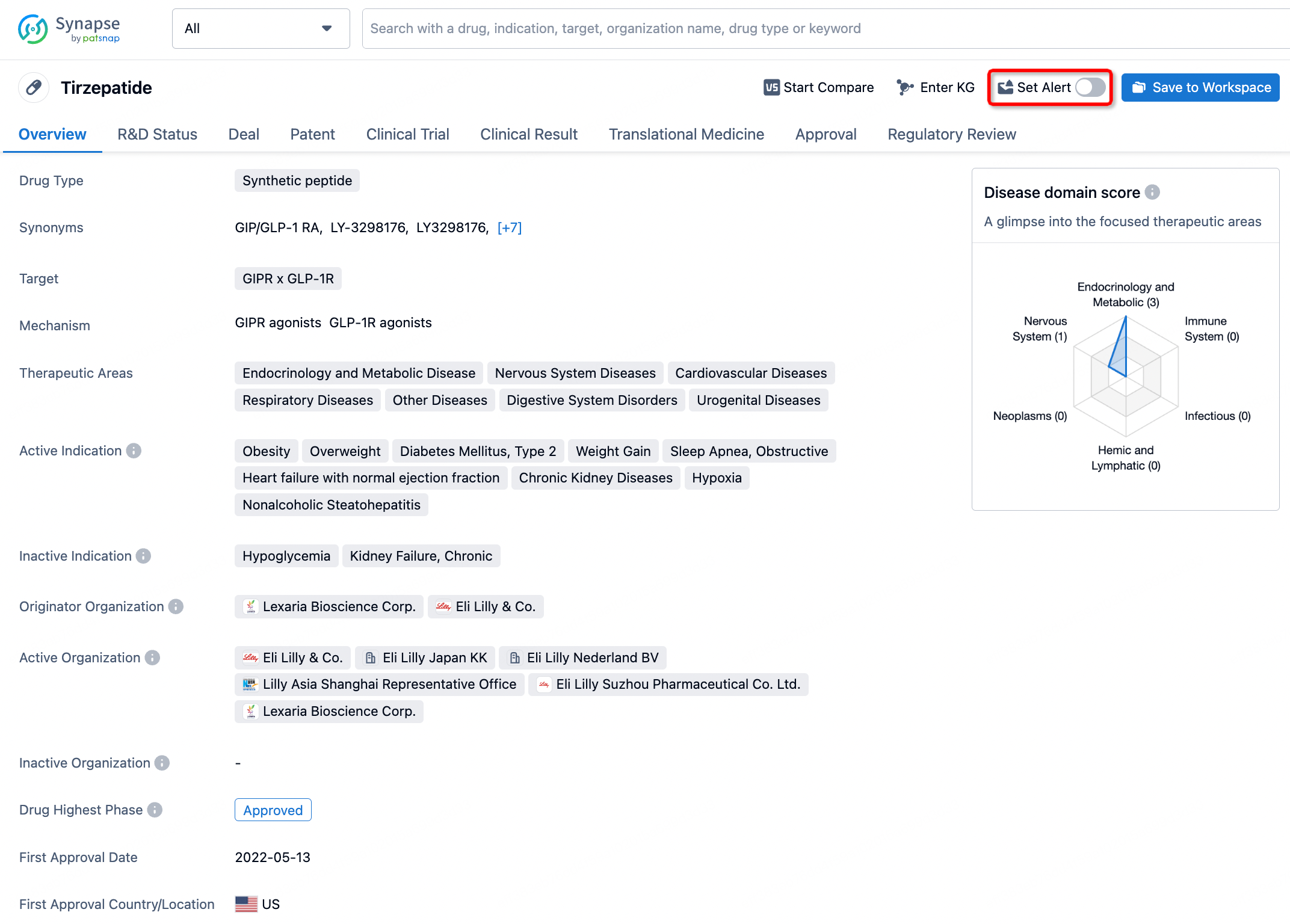
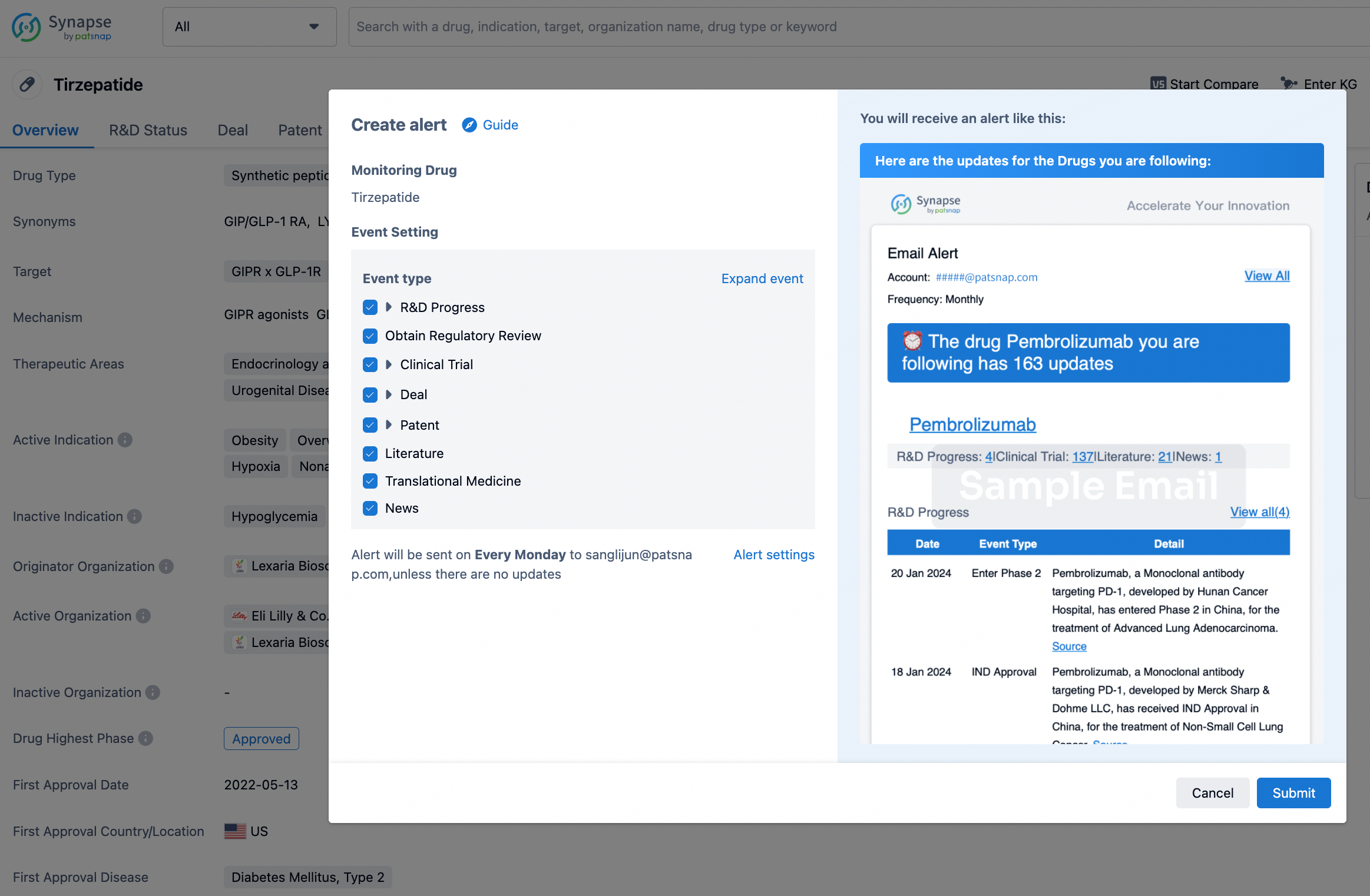
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!