Is Mobocertinib approved by the FDA?
Mobocertinib (Exkivity) received accelerated approval from the US Food and Drug Administration (FDA) on September 15, 2021. This approval provides a treatment option for patients with limited alternatives, particularly those with NSCLC caused by an EGFR exon 20 insertion mutation.
Indications and Usage
Mobocertinib is prescribed for adults with NSCLC that:
- Is locally advanced or metastatic (spread to other parts of the body) and cannot be removed by surgery.
- Has an abnormal EGFR gene caused by an exon 20 insertion mutation.
- Has progressed during or after chemotherapy containing platinum.
Mechanism of Action
EGFR is a protein that promotes cell growth and division. Mutations in the exon 20 insertion lead to abnormal cell growth, resulting in cancer. Mobocertinib targets and inhibits these specific mutations, slowing cancer cell growth and proliferation.
Administration and Dosage
The recommended dosage of mobocertinib is 160 mg taken orally once a day, with or without food. Patients are advised to swallow the capsules whole without opening, chewing, or dissolving them.
Side Effects
Mobocertinib can cause several side effects, some of which may be severe. Key side effects include:
- Serious Side Effects:
- QTc prolongation and Torsades de Pointes (irregular heartbeats that can be life-threatening).
- Severe lung problems (symptoms include trouble breathing, cough, chest pain, fever).
- Heart problems, including heart failure (symptoms include irregular heartbeats, shortness of breath, chest pain, swelling of ankles and feet, fainting).
- Diarrhea, which may be severe and cause dehydration and kidney problems.
- Common Side Effects:
- Diarrhea
- Rash
- Nausea
- Mouth sores
- Vomiting
- Decreased appetite
- Infection around the nails
- Fatigue
- Dry skin
- Muscle or bone pain
Patients should report any severe side effects to their healthcare provider immediately.
Precautions and Interactions
Before starting mobocertinib, patients should inform their healthcare provider about any pre-existing conditions, such as heart problems, electrolyte imbalances, or lung issues. It's important to avoid grapefruit products during treatment, as they can increase mobocertinib levels in the blood.
Pregnancy and Breastfeeding
Mobocertinib can harm an unborn baby. Women who can become pregnant should use effective non-hormonal birth control during treatment and for one month after the last dose. Men with partners who can become pregnant should also use effective birth control during treatment and for one week after the last dose. Breastfeeding is not recommended during treatment and for one week after the final dose.
Conclusion
Mobocertinib (Exkivity) offers a targeted therapy for adults with a specific type of NSCLC caused by EGFR exon 20 insertion mutations. Approved by the FDA on September 15, 2021, it provides a vital option for patients with limited treatment alternatives, underscoring the importance of genetic testing in cancer therapy to ensure appropriate and effective treatment.
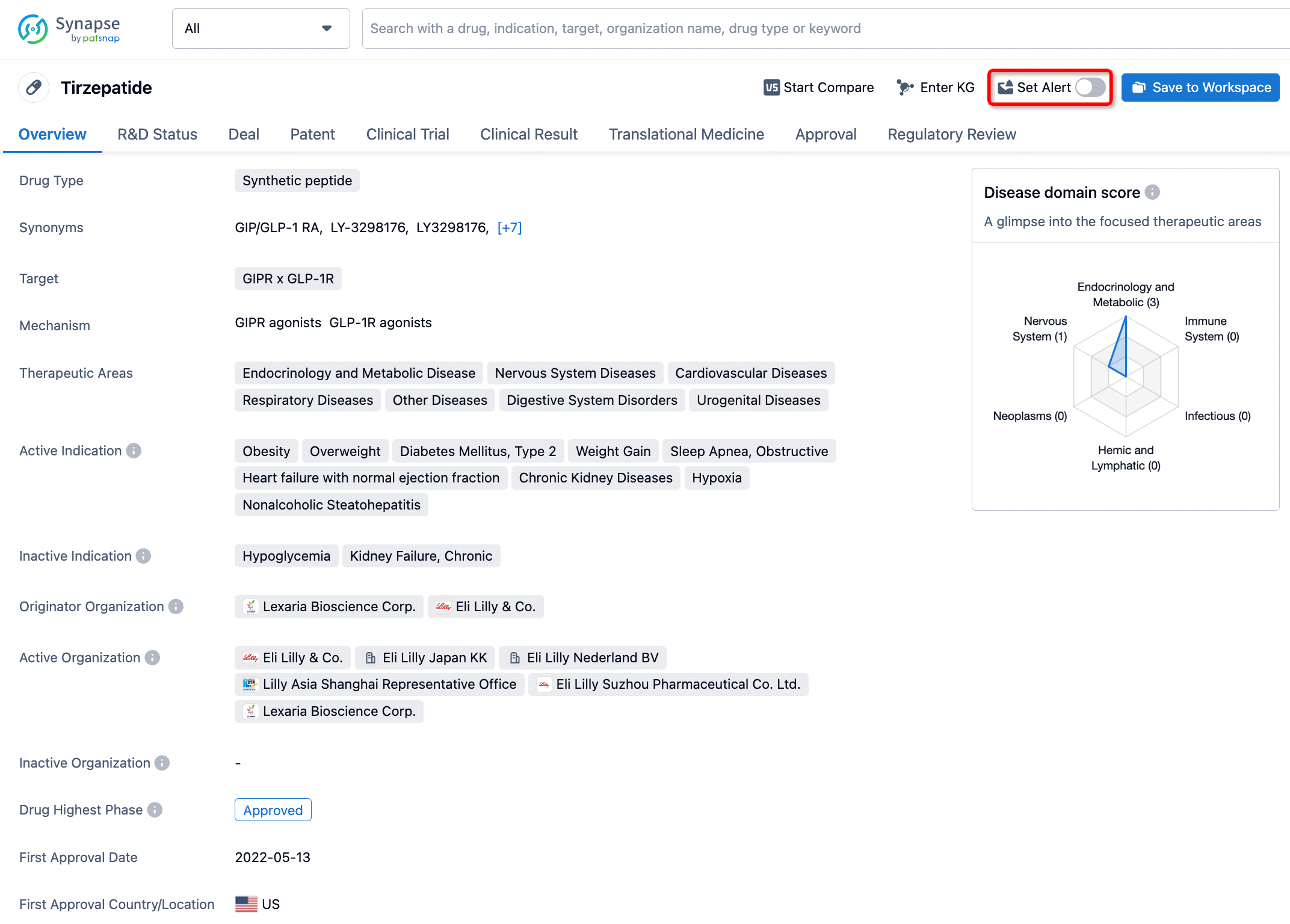
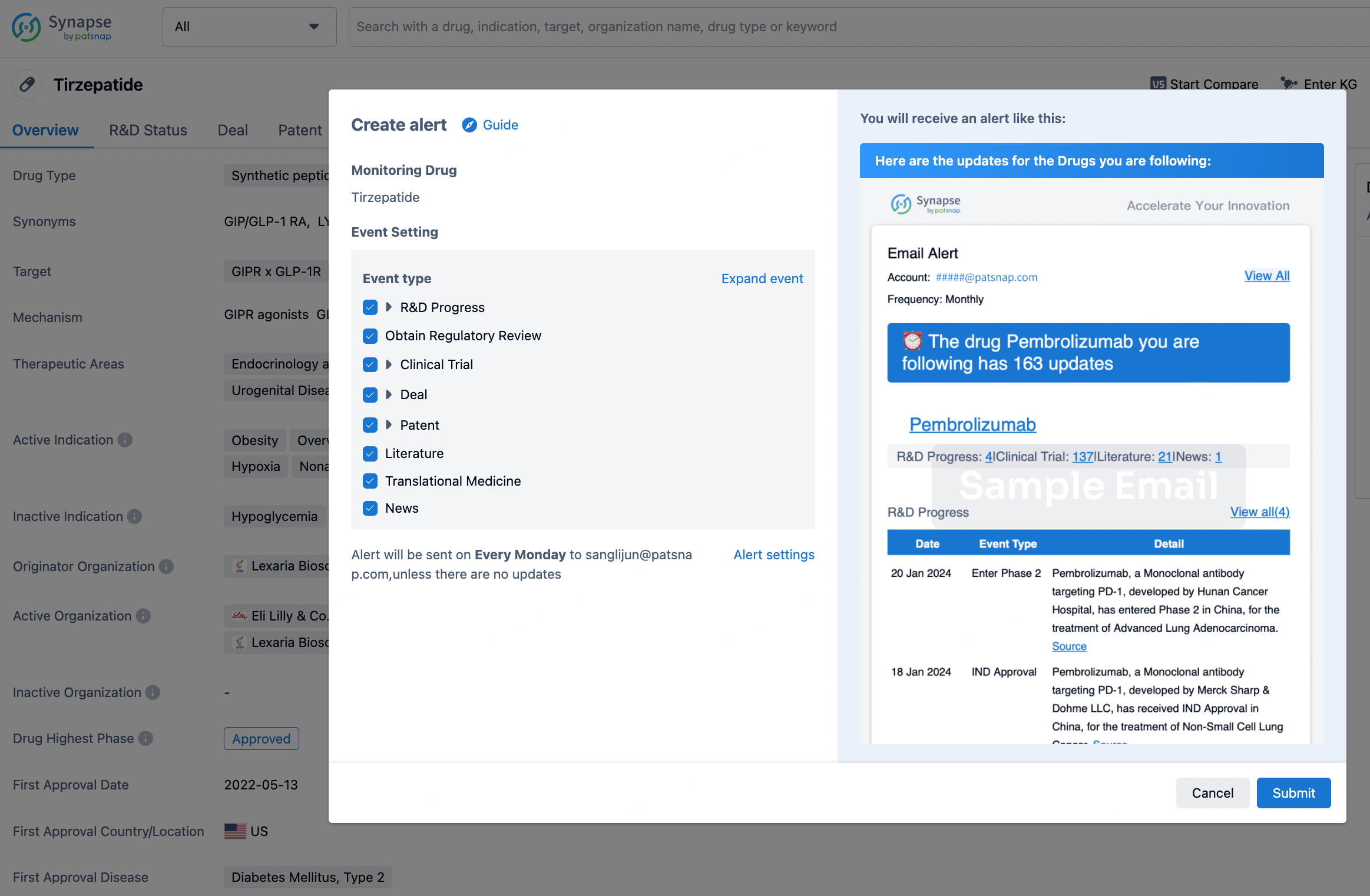
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!