Is Pemigatinib approved by the FDA?
Yes, Pemigatinib, marketed under the brand name Pemazyre, is FDA approved. The U.S. Food and Drug Administration (FDA) granted accelerated approval for Pemigatinib on April 17, 2020. This approval allows for the treatment of adults with bile duct cancer (cholangiocarcinoma) that has spread to other parts of the body or cannot be removed surgically and who have a specific genetic marker known as the FGFR2 gene fusion or rearrangement.
What is Pemigatinib?
Pemigatinib is an oral multikinase inhibitor used to treat adults with cholangiocarcinoma that is metastatic or unresectable. It is specifically indicated for use in patients whose cancer cells exhibit an FGFR2 gene fusion or rearrangement, which is determined by an FDA-approved test. Pemigatinib works by inhibiting the fibroblast growth factor receptors (FGFRs), which play a crucial role in the proliferation and survival of cancer cells.
Indications and Usage
Pemigatinib is used for treating:
- Adults with metastatic or unresectable cholangiocarcinoma with an FGFR2 gene fusion or rearrangement.
- Patients who have previously been treated with other cancer medications.
Administration and Dosage
Pemigatinib is administered orally in a 21-day treatment cycle:
- 13.5 mg taken once daily for the first 14 days, followed by a 7-day break.
Side Effects
Common side effects:
- High or low phosphate levels in the blood
- Dry eyes
- Stomach pain, nausea, vomiting, loss of appetite
- Diarrhea or constipation
- Mouth sores, dry mouth
- Joint or back pain
- Fatigue
- Nail problems
- Dry skin, hair loss
- Changes in taste
Serious side effects:
- Eye problems (pain, redness, dryness, puffiness, sensitivity to light, vision changes)
- High phosphate levels (muscle cramps, numbness, tingling around the mouth)
Precautions and Warnings
Patients should inform their healthcare provider about any pre-existing conditions, especially eye problems or vision issues. Both men and women should use effective birth control during treatment and for at least one week after the last dose due to the potential harm to an unborn baby. Breastfeeding should be avoided during treatment and for one week post-treatment.
Conclusion
Pemigatinib (Pemazyre) received FDA approval on April 17, 2020, under accelerated approval for the treatment of adults with cholangiocarcinoma exhibiting FGFR2 gene fusions or rearrangements. As a targeted therapy, Pemigatinib offers a new treatment option for patients with this specific genetic profile, especially for those whose cancer has progressed despite previous treatments.
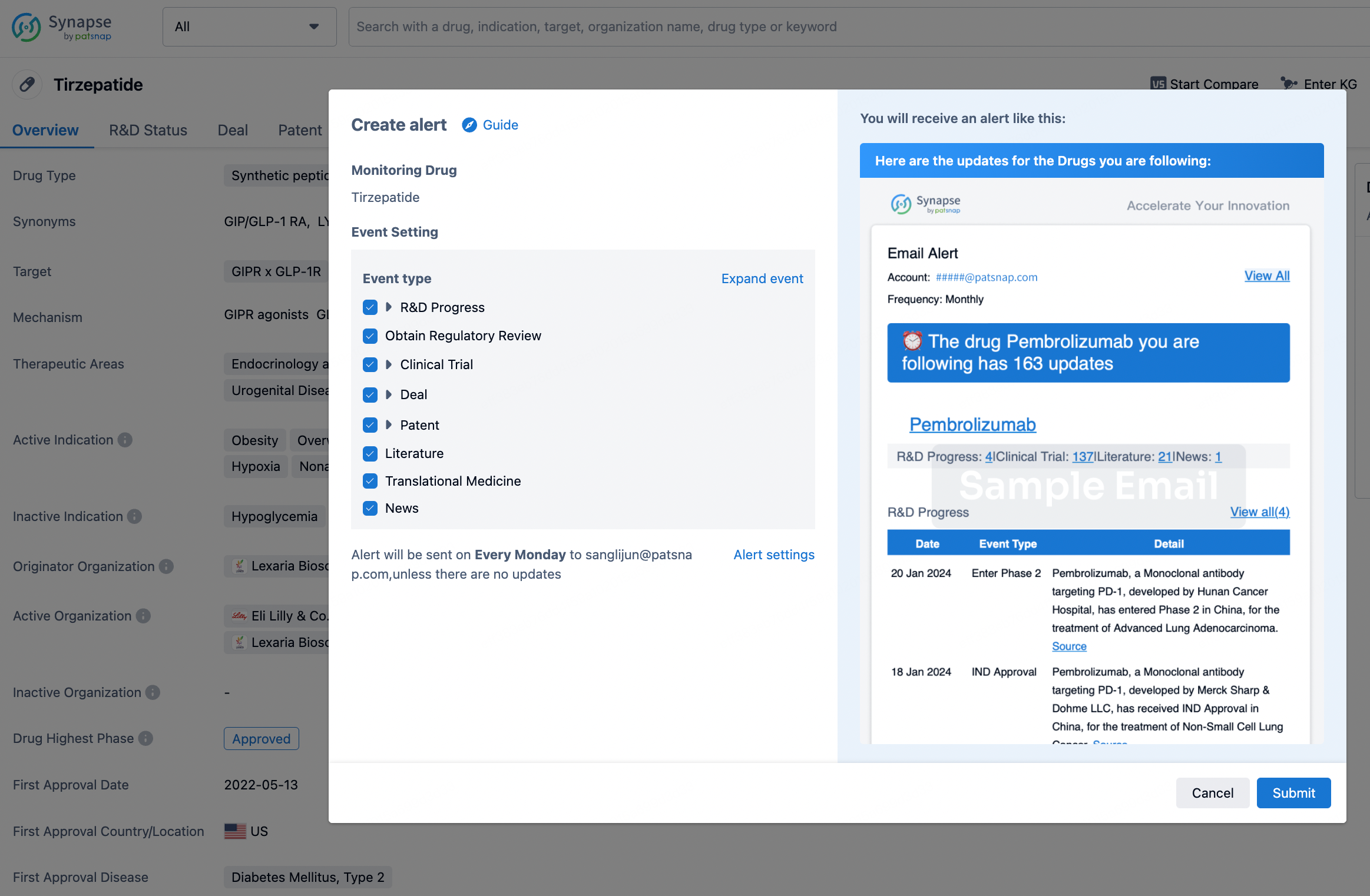
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!