Is Tazemetostat approved by the FDA?
Yes, Tazemetostat, marketed under the brand name Tazverik, is FDA approved. The U.S. Food and Drug Administration (FDA) approved Tazemetostat on January 23, 2020, for the treatment of specific types of advanced cancers, specifically epithelioid sarcoma and follicular lymphoma.
What is Tazemetostat?
Tazemetostat is an oral medication used to treat advanced epithelioid sarcoma, a rare and slow-growing type of soft tissue cancer. It is prescribed for cases where the cancer has metastasized or cannot be removed surgically. Additionally, Tazemetostat is used to treat follicular lymphoma, a type of non-Hodgkin lymphoma, particularly in patients with an EZH2 mutation or those who have not responded to at least two prior systemic therapies.
FDA Approval
The FDA granted accelerated approval to Tazemetostat based on clinical study results that showed a significant number of patients responding to the treatment. However, further studies are needed to confirm the clinical benefits of Tazemetostat.
How is Tazemetostat Administered?
Tazemetostat is administered orally in tablet form. The typical dosage for adults and children aged 16 and older is:
- Advanced Epithelioid Sarcoma: 800 mg twice daily until disease progression or unacceptable toxicity occurs.
- Follicular Lymphoma: 800 mg twice daily, particularly for those with relapsed or refractory disease and an EZH2 mutation.
Side Effects
Tazemetostat can cause a range of side effects, some of which may be severe. Common side effects include:
- Nausea and vomiting
- Loss of appetite
- Constipation
- General pain
- Fatigue
Serious side effects may include unusual tiredness, bone pain, and low blood cell counts, which could manifest as fever, chills, mouth sores, easy bruising, unusual bleeding, pale skin, or shortness of breath. Patients experiencing these symptoms should seek immediate medical attention.
Warnings and Precautions
Tazemetostat may increase the risk of developing bone marrow disorders or other cancers, such as leukemia or lymphoma. Patients are advised to have a negative pregnancy test before starting treatment and to use effective nonhormonal birth control during and for six months after treatment, as the drug can harm an unborn baby. Hormonal contraceptives may be less effective when used alongside Tazemetostat, so barrier methods are recommended.
Usage Instructions
Patients should follow their healthcare provider's instructions carefully, taking Tazemetostat tablets whole without crushing, chewing, or breaking them. The medication can be taken with or without food, but patients should avoid grapefruit products and St. John's wort, as these can interfere with the drug's effectiveness.
Conclusion
Tazemetostat (Tazverik) represents an important advancement in the treatment of certain advanced cancers, particularly epithelioid sarcoma and follicular lymphoma. Its FDA approval marks a significant step forward, offering hope to patients with these challenging conditions. As with any medication, it is crucial for patients to adhere to their healthcare provider's guidance and report any side effects promptly to ensure the best possible outcomes.
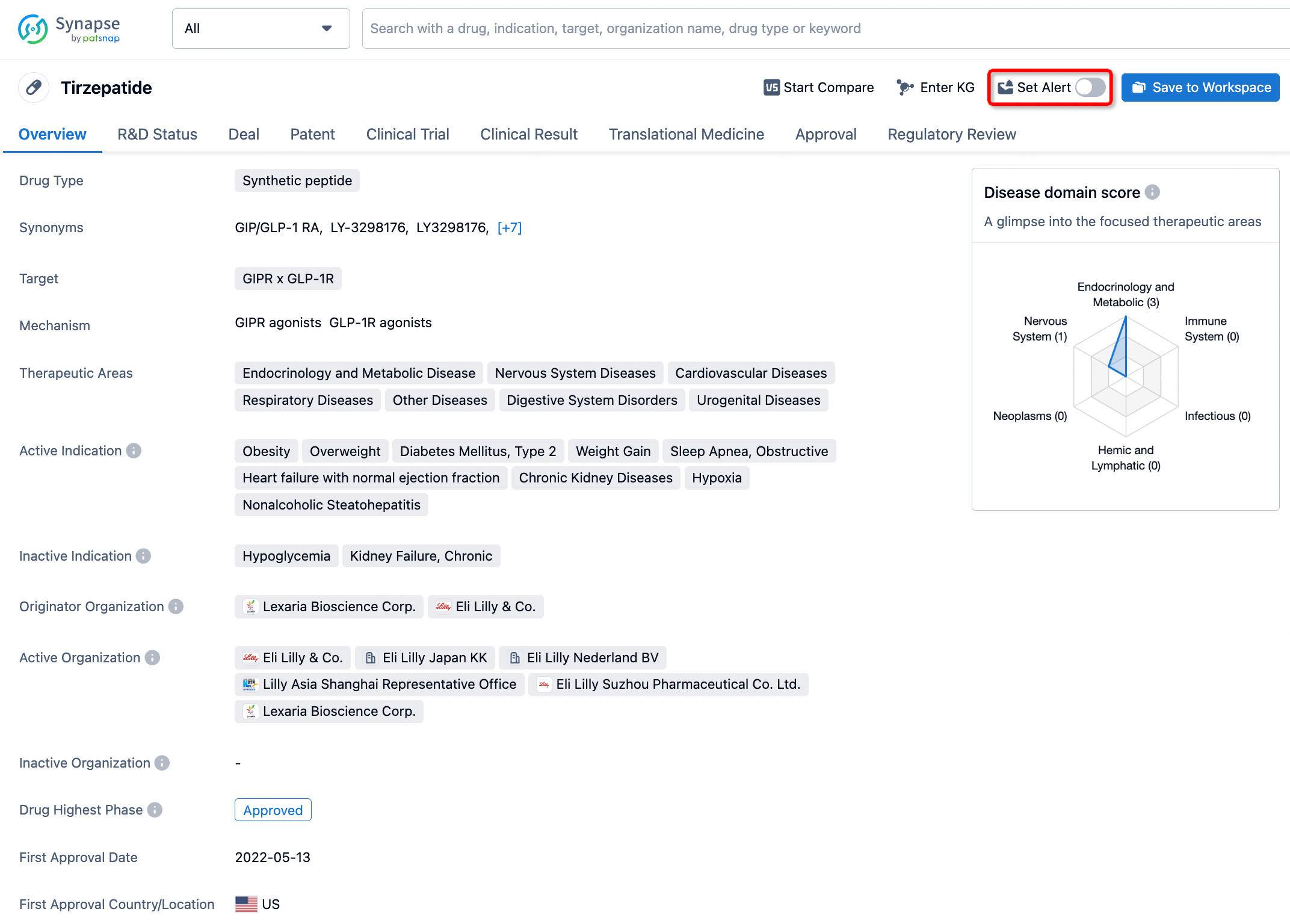
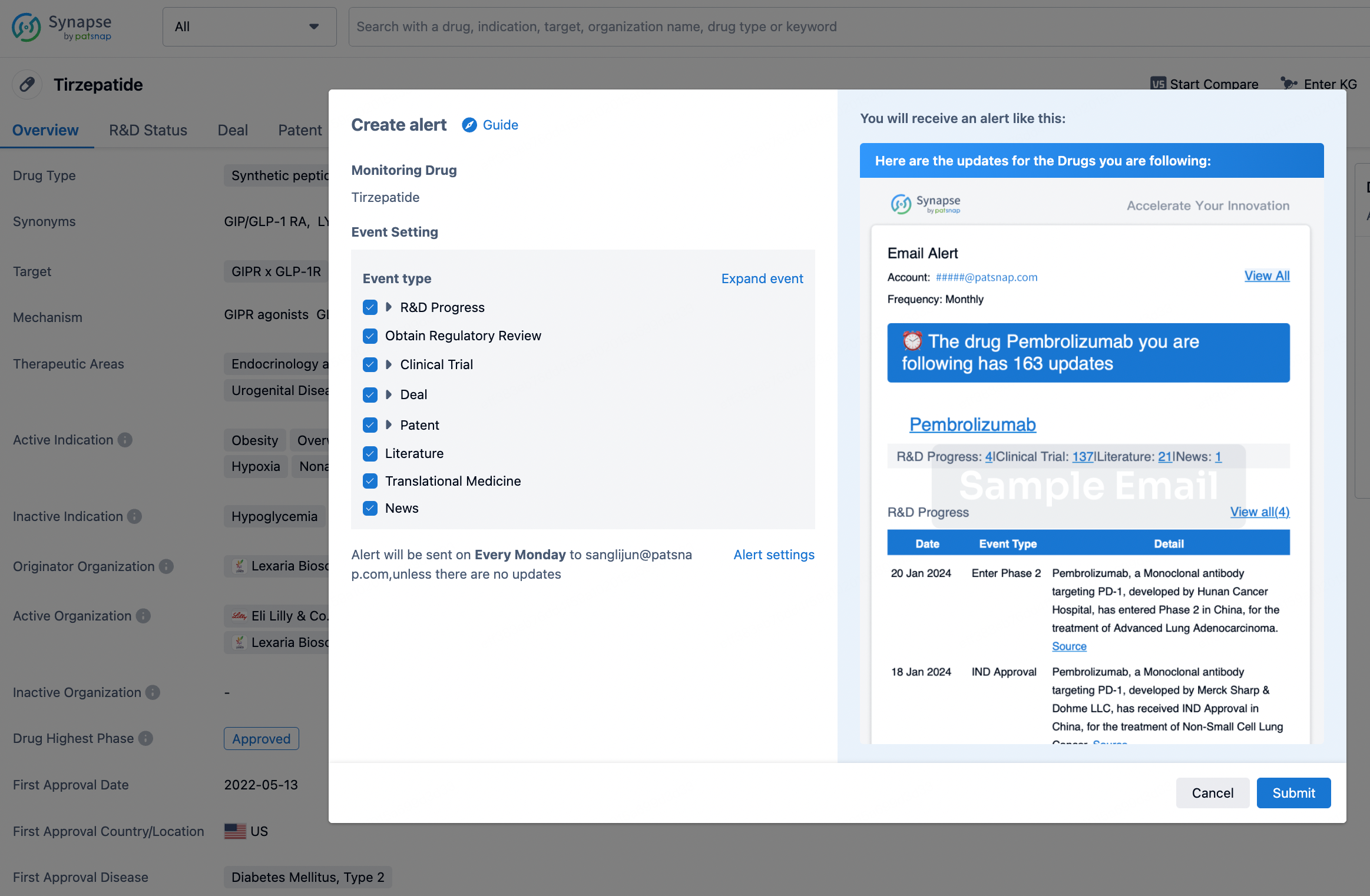
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!