Is Viloxazine approved by the FDA?
Viloxazine, marketed under the brand name Qelbree, is an oral medication used to treat attention deficit hyperactivity disorder (ADHD) in children aged 6 to 17 years. Viloxazine (Qelbree) was approved by the U.S. Food and Drug Administration (FDA) on April 2, 2021. This approval marked the introduction of a novel treatment option for pediatric ADHD, providing an alternative to traditional stimulant medications.
Mechanism of Action
Viloxazine works by inhibiting the reuptake of norepinephrine, a neurotransmitter, thereby increasing its levels in the brain. This action helps improve attention and decrease impulsiveness and hyperactivity in patients with ADHD.
Usage and Administration
Dosage Form: Viloxazine is available as an extended-release oral capsule in three strengths: 100 mg, 150 mg, and 200 mg.
Dosage Instructions:
- Children (6 to 11 years): The initial dose is 100 mg once daily, which may be increased weekly by 100 mg based on response and tolerability, up to a maximum of 400 mg per day.
- Adolescents (12 to 17 years): The initial dose is 200 mg once daily, which may be increased to 400 mg after one week based on response and tolerability.
Administration:
- Viloxazine can be taken with or without food.
- The capsule should be swallowed whole; if the patient cannot swallow it, the capsule can be opened, and the contents mixed with applesauce. The mixture should be swallowed immediately without chewing.
- Regular monitoring of heart rate and blood pressure is recommended during treatment.
Side Effects and Warnings
Common Side Effects:
- Drowsiness
- Fatigue
- Irritability
- Insomnia
- Nausea
- Vomiting
- Loss of appetite
Serious Side Effects:
- Mood or behavior changes, including anxiety, panic attacks, and suicidal thoughts
- Symptoms of a manic episode, such as racing thoughts, increased energy, and risk-taking behavior
- Weight loss or failure to gain weight in children
Warnings:
- Some patients may experience suicidal thoughts while taking viloxazine. It is important to monitor for changes in mood or symptoms and report any new or worsening symptoms to a doctor.
- Viloxazine should not be used with MAO inhibitors or within 14 days of stopping an MAO inhibitor due to the risk of serious drug interactions.
- Inform your doctor if you have a history of depression, bipolar disorder, psychosis, heart problems, high blood pressure, liver disease, or severe kidney disease.
- Viloxazine may be harmful during pregnancy. Pregnant women should consult their doctor immediately.
Conclusion
With its unique mechanism of action and extended-release formulation, viloxazine provides an alternative to traditional ADHD medications, helping to manage symptoms and improve the quality of life for young patients.
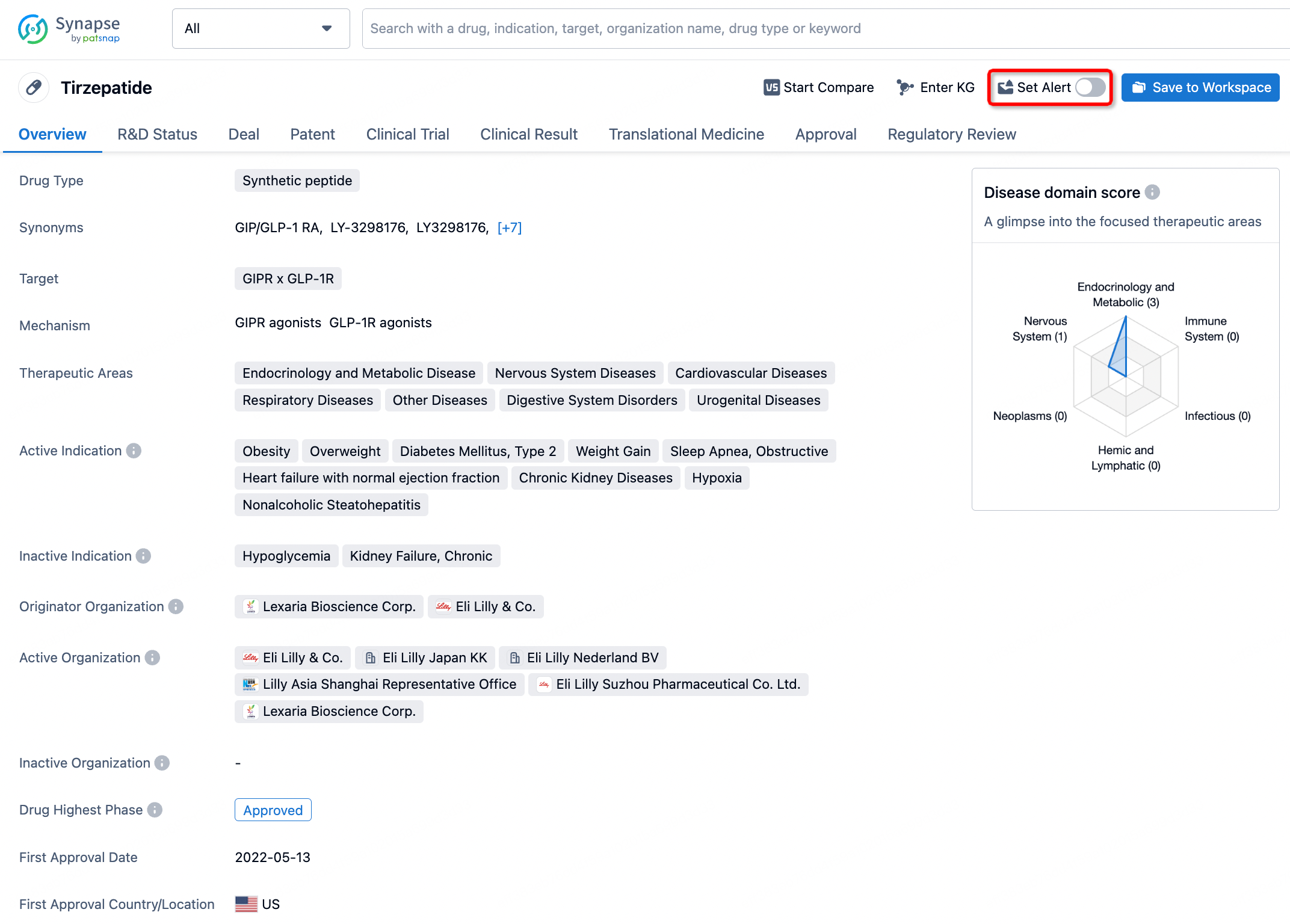
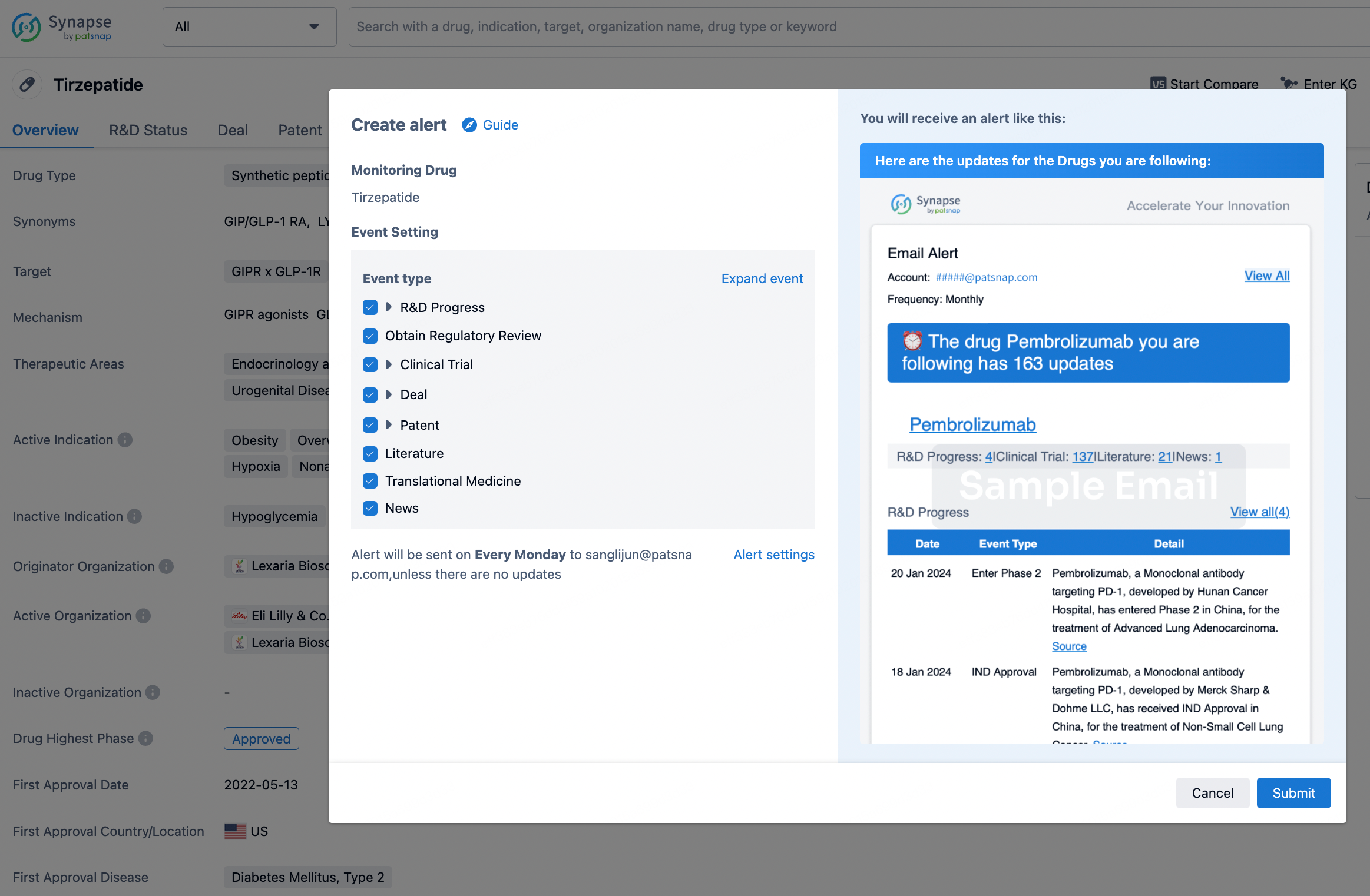
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!