Kymera Therapeutics Starts Phase 1 Trial of KT-621 with First Participant Dosed
Kymera Therapeutics, Inc. (NASDAQ: KYMR), a biopharmaceutical company in the clinical development stage that is pioneering a novel category of small molecule therapies focused on targeted protein degradation (TPD), has announced the commencement of dosing in a Phase 1 clinical trial in the United States. This trial assesses KT-621, a highly effective and selective oral degrader of STAT6, among healthy adult participants. The company anticipates sharing data from the Phase 1 trial in the first half of 2025.
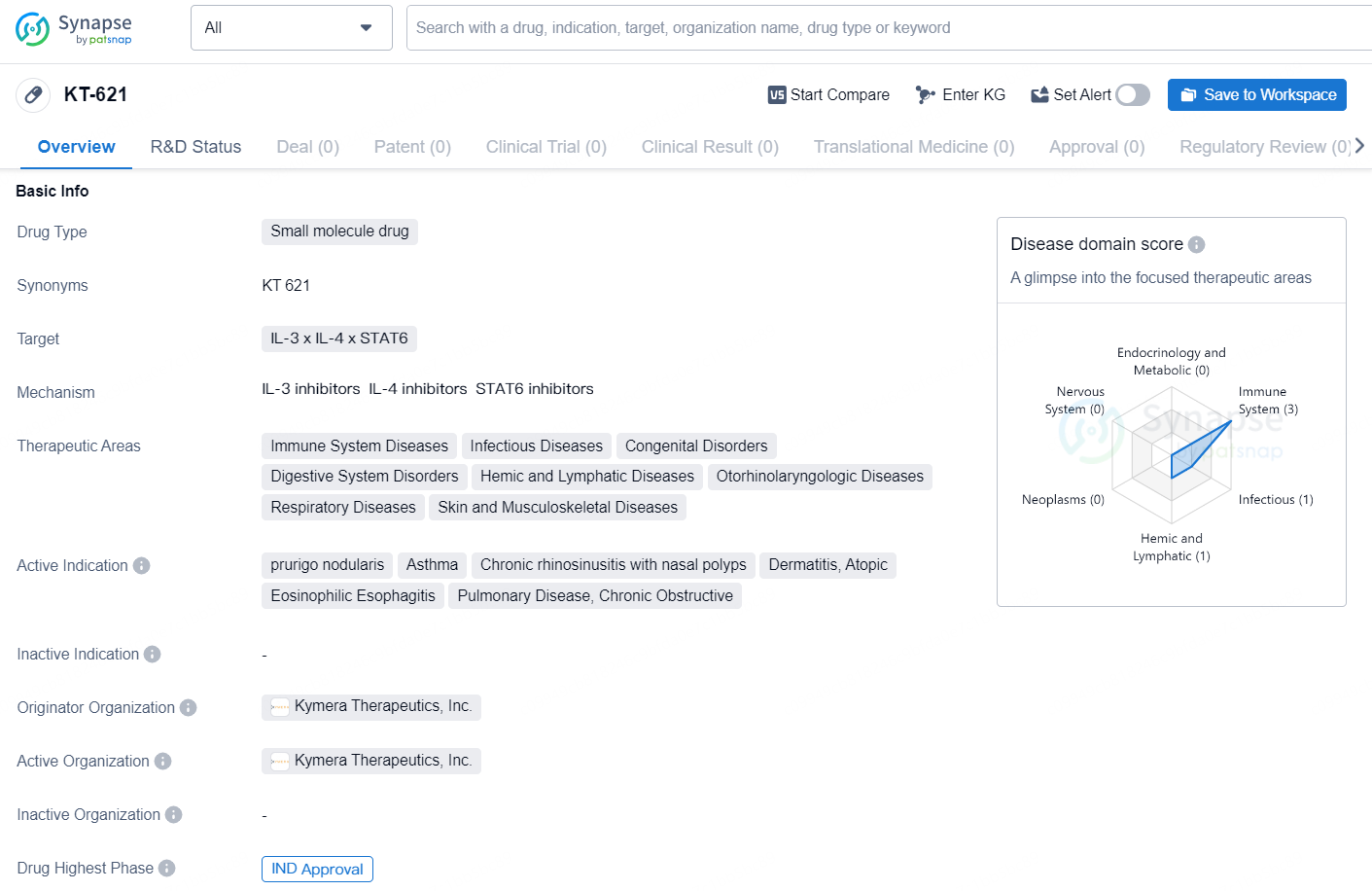
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“KT-621 is the first oral medication aimed at targeting STAT6 to enter clinical trials, highlighting Kymera's expertise in developing therapies for previously untreatable disease-related proteins that have been challenging for current treatments,” stated Nello Mainolfi, PhD, Founder, President, and CEO of Kymera Therapeutics. “We are optimistic that KT-621 can offer the convenience of a once-daily oral dosage while potentially delivering biologic-like effects for patients affected by widespread allergic and atopic disorders across the globe. Our extensive preclinical data for KT-621 has shown that degrading STAT6 achieves a comparable level of pathway inhibition as the injectable IL-4Rα antibody dupilumab while demonstrating an excellent safety profile. We are eager to move forward with this fully-owned asset through the Phase 1 study involving healthy volunteers, followed by trials in patient populations.”
The Phase 1 study will assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of KT-621 administered orally to healthy participants. The trial consists of double-blind, placebo-controlled single ascending dose (SAD) and multiple ascending dose (MAD) groups.
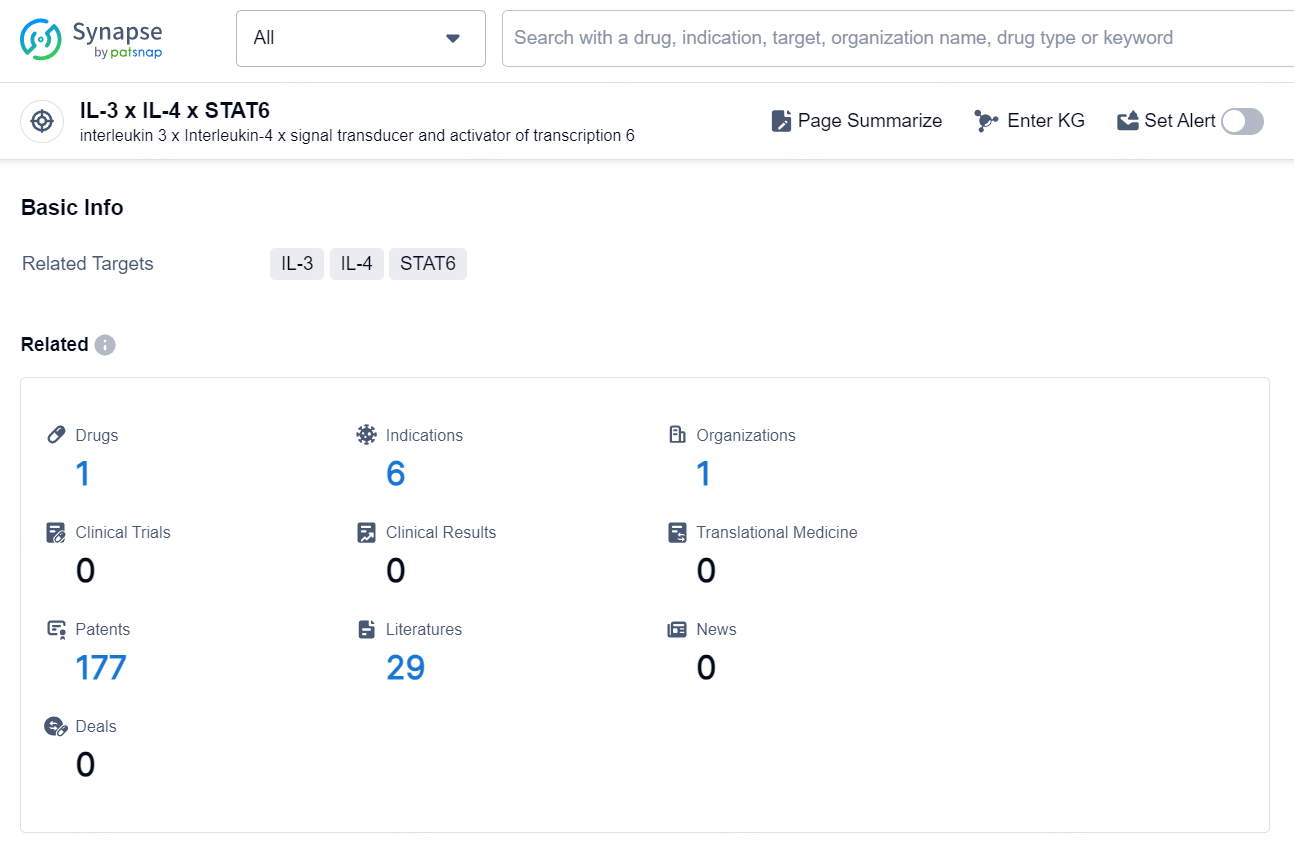
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 29, 2024, there are 1 investigational drug for the IL-3 x IL-4 x STAT6 targets, including 6 indications, 1 R&D institution involved,and as many as 177 patents.
KT-621 is a small molecule drug developed by Kymera Therapeutics, Inc. that targets interleukin 3 (IL-3), interleukin 4 (IL-4), and signal transducer and activator of transcription 6 (STAT6). The drug falls within the therapeutic areas of immune system diseases, infectious diseases, congenital disorders, digestive system disorders, hemic and lymphatic diseases, otorhinolaryngologic diseases, respiratory diseases, and skin and musculoskeletal diseases. Its active indications include prurigo nodularis, asthma, chronic rhinosinusitis with nasal polyps, dermatitis (atopic), eosinophilic esophagitis, and chronic obstructive pulmonary disease.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!