Kodiak Sciences Initiates Phase 3 GLOW2 Study on Tarcocimab Tedromer for Diabetic Retinopathy
Kodiak Sciences Inc., a biopharmaceutical enterprise focused on the research, development, and commercialization of innovative therapies for various retinal diseases, disclosed today the initial treatment of diabetic retinopathy patients in the randomized, double-blind Phase 3 GLOW2 trial of tarcocimab tedromer.
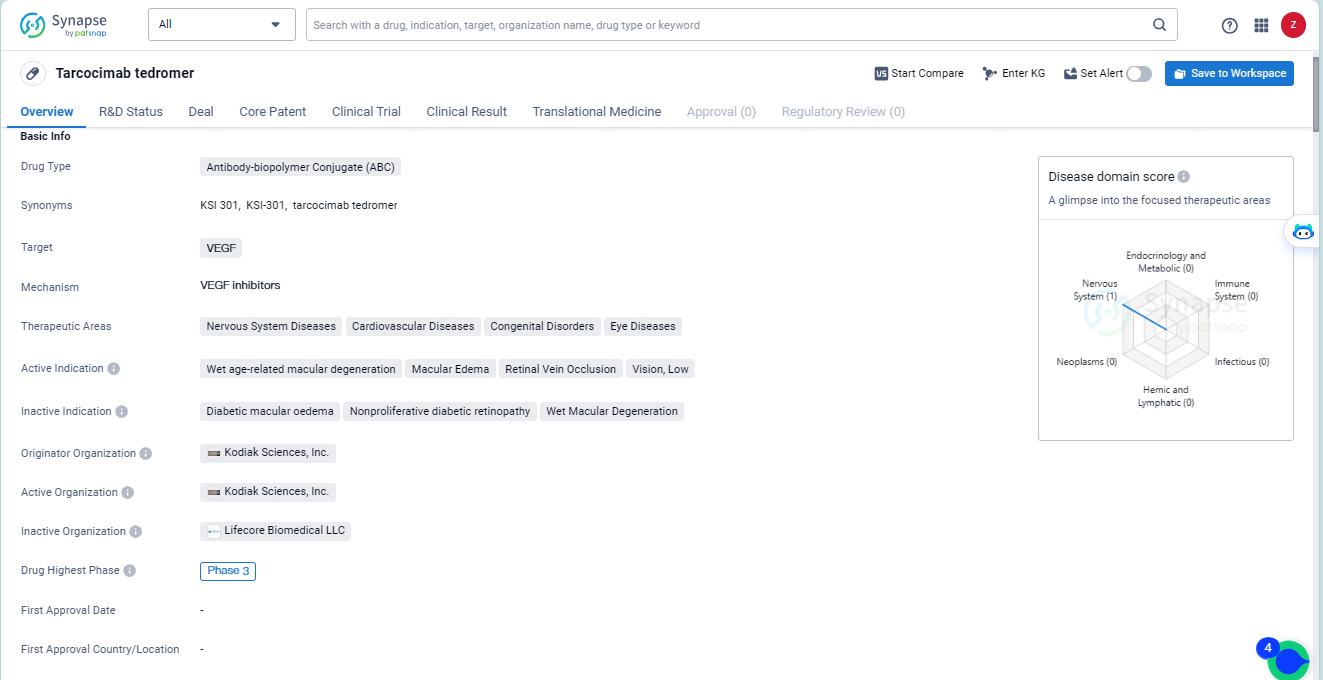
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
GLOW2 represents the second Phase 3 trial dedicated to evaluating tarcocimab for diabetic retinopathy, where every participant receiving the experimental treatment will be administered tarcocimab at expanded dosing schedules, incorporating intervals of up to six months for every subject. This study's structure follows the previously conducted successful GLOW1 trial, wherein patients treated with tarcocimab over a session lasting 48 weeks displayed a 29-fold enhancement in response with a ≥2-step improvement in DRSS, coupled with an 89% reduction in the likelihood of developing serious vision-related issues.
In alignment with Kodiak's history of efficiently managing multiple critical trials simultaneously within the tarcocimab program, GLOW2 progresses swiftly in terms of activating sites, screening new participants, and performing randomizations, aiming to finalize participant enrollment by year-end.
Dr. Victor Perlroth, CEO of Kodiak, explained regarding the findings from GLOW1, “The data definitively demonstrated that tarcocimab, when administered at prolonged dosing intervals including a six-month cycle for each patient, not only treats the existing condition but also prevents its progression. This stands out in the arena of long-acting treatments, as many merely aim to keep the disease unchanged rather than improving it.”
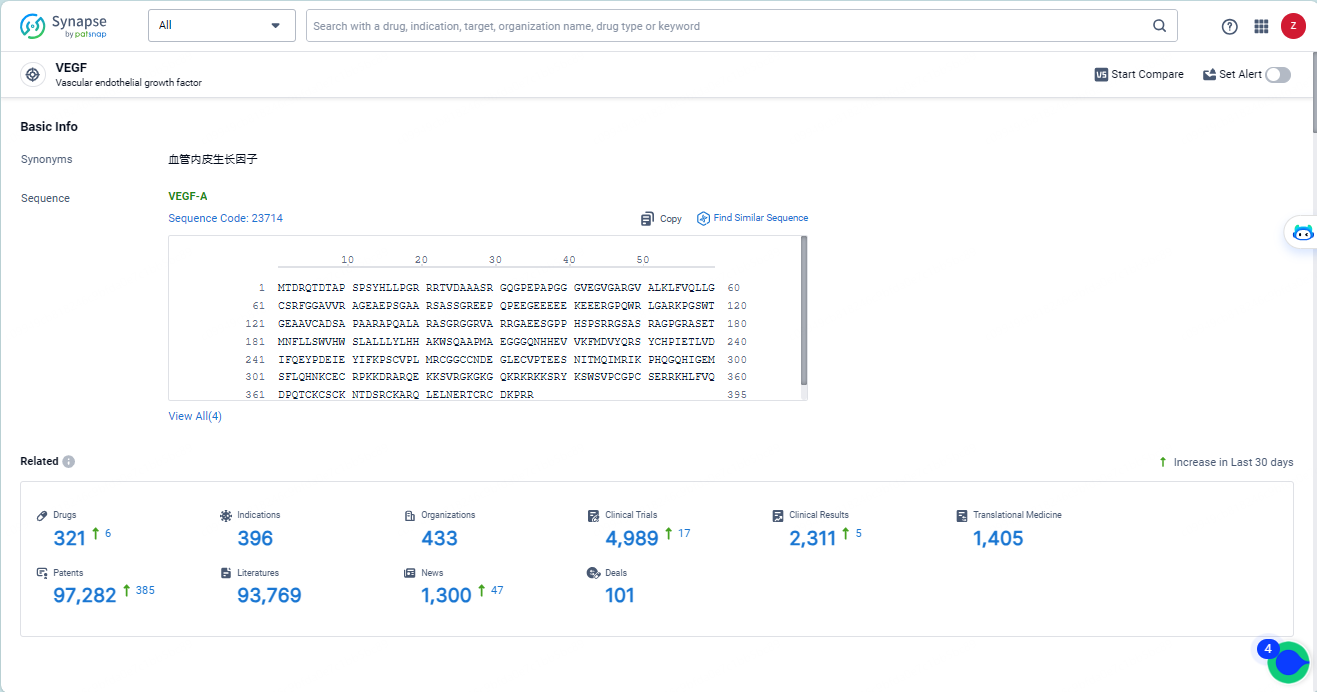
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 14, 2024, there are 321 investigational drugs for the VEGF target, including 396 indications, 433 R&D institutions involved, with related clinical trials reaching 4989, and as many as 97282 patents.
Tarcocimab tedromer is an antibody-biopolymer conjugate drug developed by Kodiak Sciences, Inc. It targets VEGF and aims to treat various diseases related to the nervous system, cardiovascular system, congenital disorders, and eye diseases. The drug has reached Phase 3 globally, but its development in China has been discontinued.