LegChem Bio has entered into a licensing deal for their Trop2-focused antibody-drug conjugate known as LCB84
LegoChem Biosciences, Inc., revealed on the 22nd of December its new licensing partnership with Janssen Biotech, Inc., part of the Johnson & Johnson family of companies, for the advancement and marketing of their specialized therapeutic, LCB84, an antibody-drug conjugate targeting Trop2.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Pursuant to the contractual arrangement, LCB confers upon Janssen a sole and universal entitlement to proceed with the research, development, and marketing of LCB84. LCB stands to receive as much as USD 1.7 billion consisting of several financial components, namely a preliminary disbursement of USD 100 million, a payment of USD 200 million upon exercising the option, in addition to potential payments tied to developmental, regulatory, and sales milestones, paired with escalating royalties predicated on global sales.
A Joint effort will persist throughout the ongoing early-stage clinical research; thereafter, upon exercising the option, Janssen will become the exclusive party in charge of the clinical advancement and market introduction of the product. The ADC, LCB84, harnesses the novel Trop2 axis and embodies LCB's cutting-edge ADC technological framework, along with a Trop2 antibody, under a licensing agreement from Mediterranea Theranostic, S.r.l. It is currently under investigation in an early-phase clinical trial in the United States.
Showcasing a novel targeted approach, the ADC binds a distinct cleavable variant of the Trop2 antigen, which shows elevated expression within tumor cells. Preclinical studies reveal that LCB84 stands out due to its safety and efficacy profile when evaluated over a diverse array of cancer types, enhancing its potential as a therapeutic ADC aimed at Trop2.
LCB's chief executive, Yong-Zu Kim, stated, "We are delighted to be in collaboration with Janssen regarding LCB84, marking our inaugural internally generated ADC in clinical development." He further expressed eagerness in fortifying LCB's international clinical development and fostering additional ADC initiatives towards clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
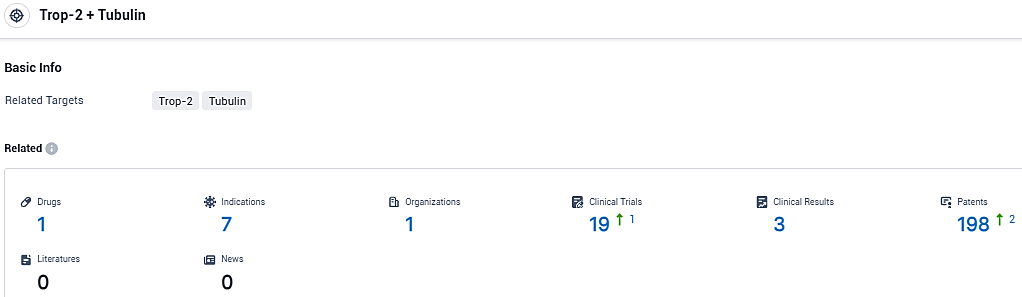
According to the data provided by the Synapse Database, As of December 30, 2023, there are 1 investigational drugs for the Trop-2 and Tubulin target, including 7 indications, 1 R&D institutions involved, with related clinical trials reaching 19, and as many as 198 patents.
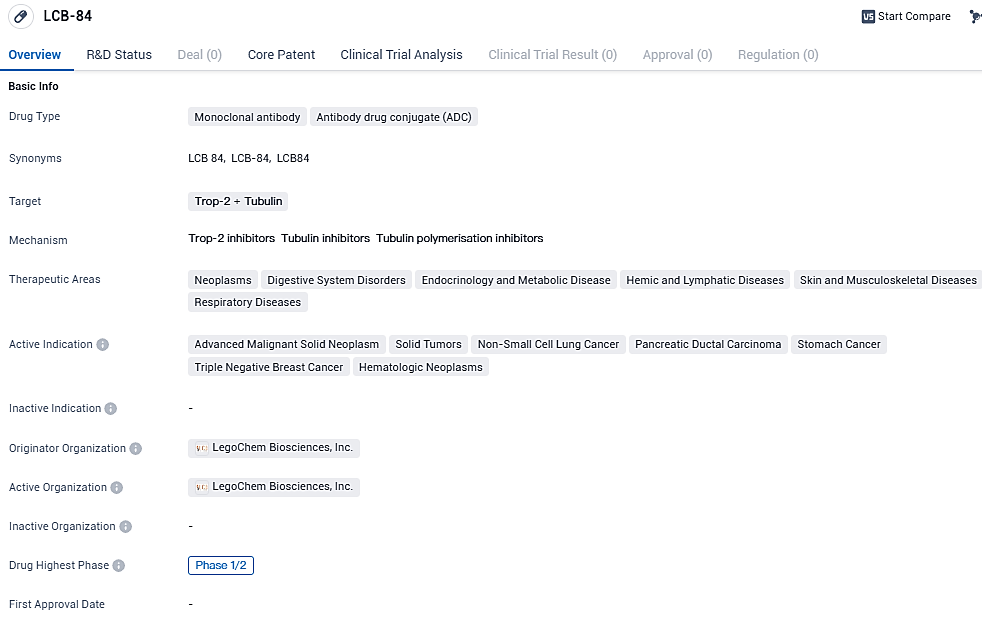
LCB-84 targets Trop-2 and Tubulin proteins and shows potential in treating various neoplastic diseases, digestive system disorders, endocrinology and metabolic diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, and respiratory diseases. The drug is currently in Phase 1/2 of development, indicating its progress in clinical trials. Further research is needed to determine its safety, efficacy, and potential impact on patient outcomes.