Understanding 5-HT1A receptor agonists and Methods to Keep Abreast of Their Recent Developments
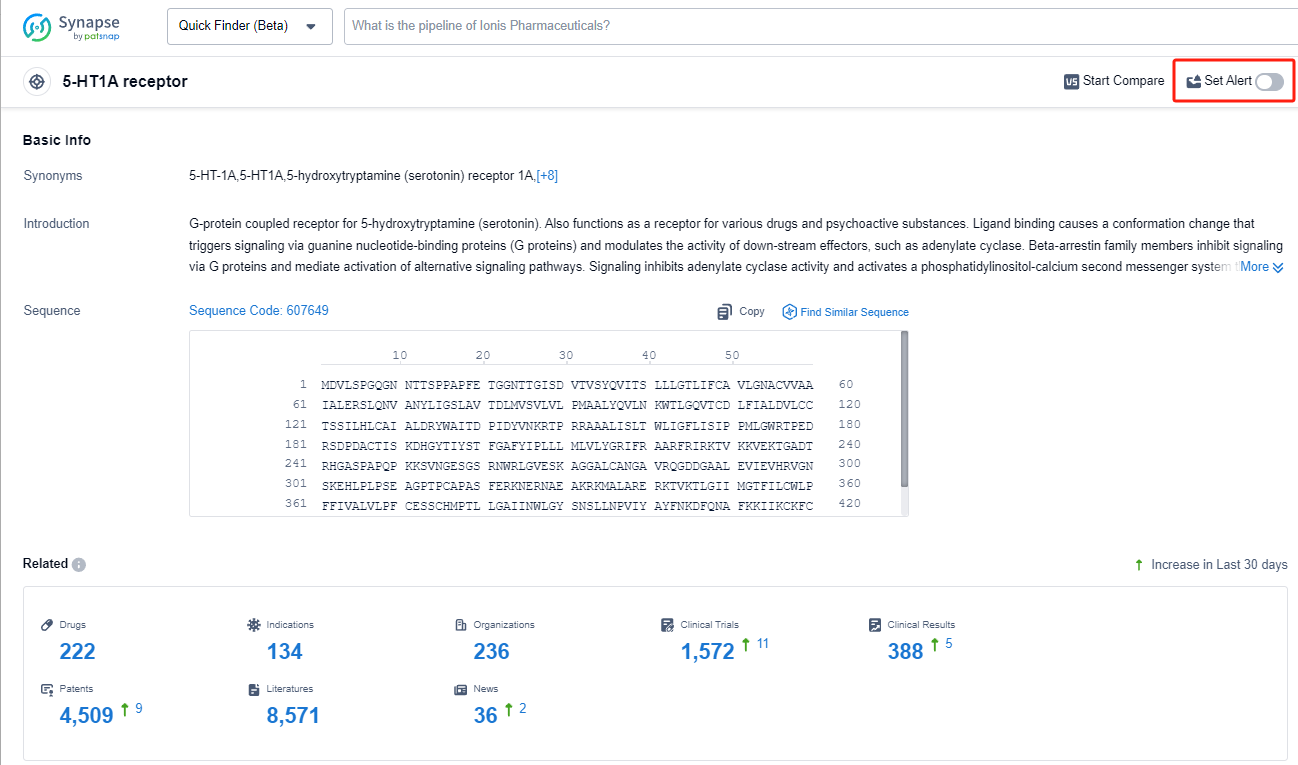
The 5-HT1A receptor is a subtype of serotonin receptor found in the human body. It plays a crucial role in regulating various physiological and psychological processes. Activation of the 5-HT1A receptor by serotonin, a neurotransmitter, leads to inhibitory effects on neuronal activity, resulting in the modulation of mood, anxiety, and stress responses. This receptor is also involved in the regulation of sleep, appetite, and pain perception. Dysfunction of the 5-HT1A receptor has been implicated in various psychiatric disorders, including depression, anxiety disorders, and schizophrenia. Understanding the role of the 5-HT1A receptor is essential for the development of novel therapeutic interventions targeting these conditions.
The development of 5-HT1A receptor agonists has been a significant advancement in the field of medicine. The first 5-HT1A receptor agonist, buspirone, was approved by the FDA in 1986 for the treatment of generalized anxiety disorder. Since then, several other 5-HT1A receptor agonists have been developed, including gepirone, tandospirone, and flesinoxan.
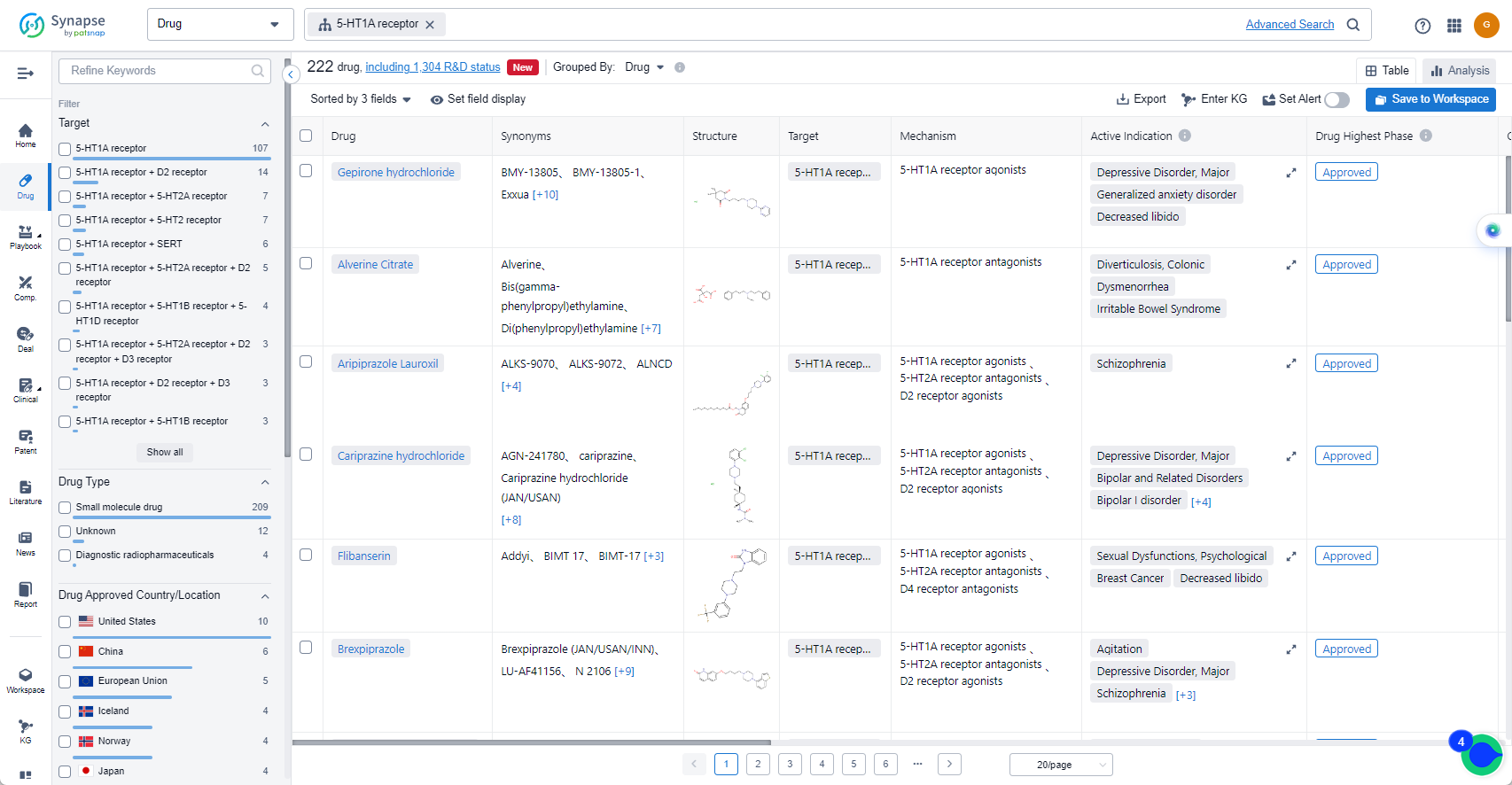
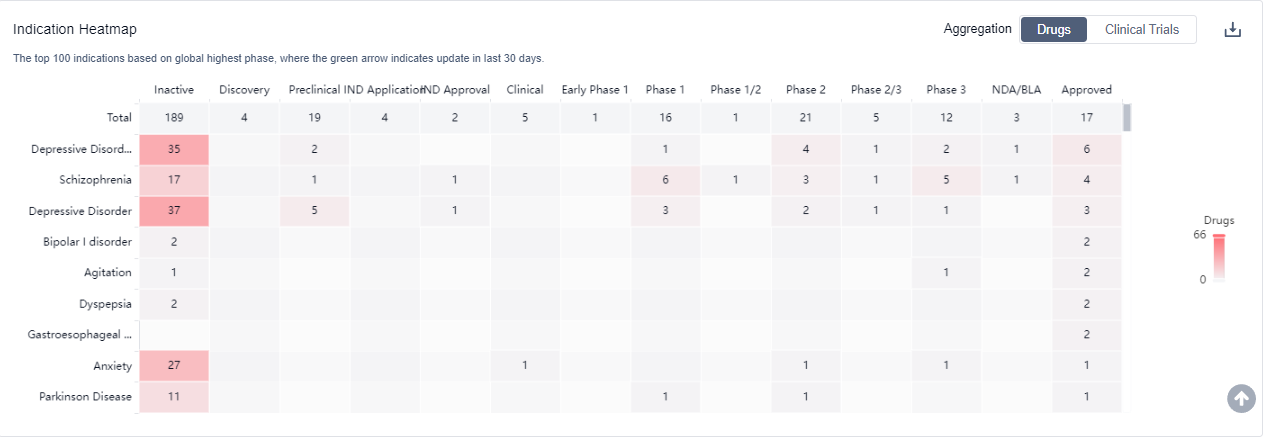
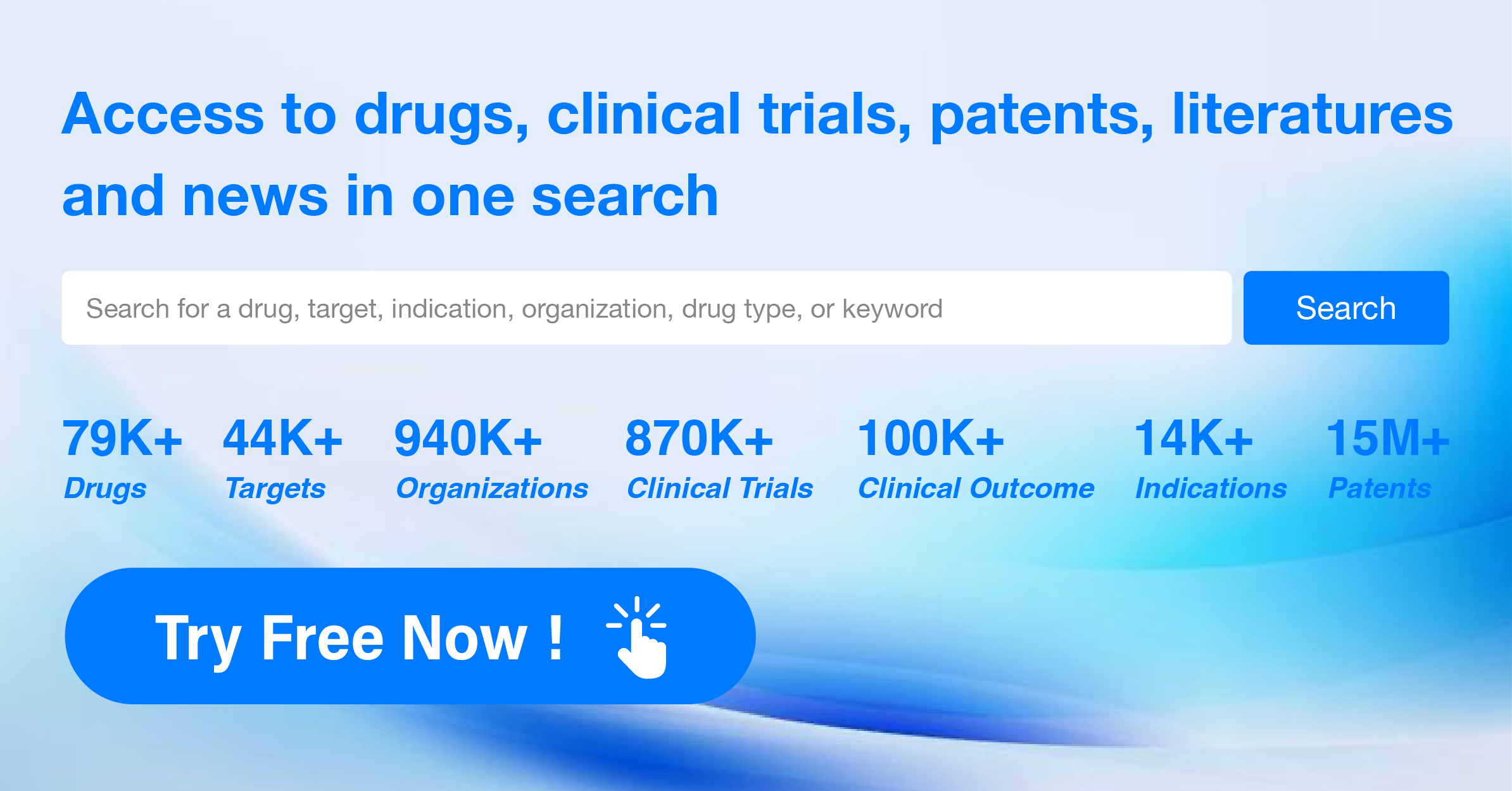
The analysis of the target 5-HT1A receptor reveals that Otsuka Holdings Co., Ltd., AbbVie, Inc., and Lundbeck Foundation are the companies growing fastest and have made significant R&D progress. The approved indications for drugs targeting the 5-HT1A receptor include Depressive Disorder, Major, Schizophrenia, Bipolar I disorder, and others. Small molecule drugs are progressing most rapidly, indicating a focus on this drug type. The United States, China, and the European Union are the countries/locations developing fastest, with progress also observed in China. Overall, the target 5-HT1A receptor presents a competitive landscape with potential for future development in various therapeutic areas.
How do they work?
5-HT1A receptor agonists are a type of drugs that specifically target and activate the 5-HT1A receptors in the brain. These receptors are a subtype of serotonin receptors, which are involved in regulating various physiological and behavioral processes.
From a biomedical perspective, 5-HT1A receptor agonists have been studied and used in the treatment of several conditions, particularly those related to the central nervous system. By binding to and activating the 5-HT1A receptors, these drugs can modulate serotonin levels and signaling pathways, leading to various therapeutic effects.
Some potential indications for 5-HT1A receptor agonists include the treatment of anxiety disorders, depression, and certain types of pain. These drugs may help alleviate symptoms by promoting serotonin release, which can enhance mood, reduce anxiety, and modulate pain perception.
It's worth noting that the specific mechanism of action and effects of 5-HT1A receptor agonists can vary depending on the particular drug and its pharmacokinetics. Therefore, it is important to consult with a healthcare professional for detailed information about the specific drug and its indications, contraindications, and potential side effects.
List of 5-HT1A receptor Agonists
The currently marketed 5-HT1A receptor agonists include:
- Gepirone hydrochloride
- Aripiprazole Lauroxil
- Cariprazine hydrochloride
- Flibanserin
- Brexpiprazole
- Vortioxetine Hydrobromide
- Vilazodone Hydrochloride
- Aripiprazole
- Tandospirone Citrate
- Cinitapride Tartrate
For more information, please click on the image below.
What are 5-HT1A receptor agonists used for?
5-HT1A receptor agonists are under investigation for indications that include the treatment of anxiety disorders, depression, and certain types of pain. For more information, please click on the image below to log in and search.
How to obtain the latest development progress of 5-HT1A receptor agonists?
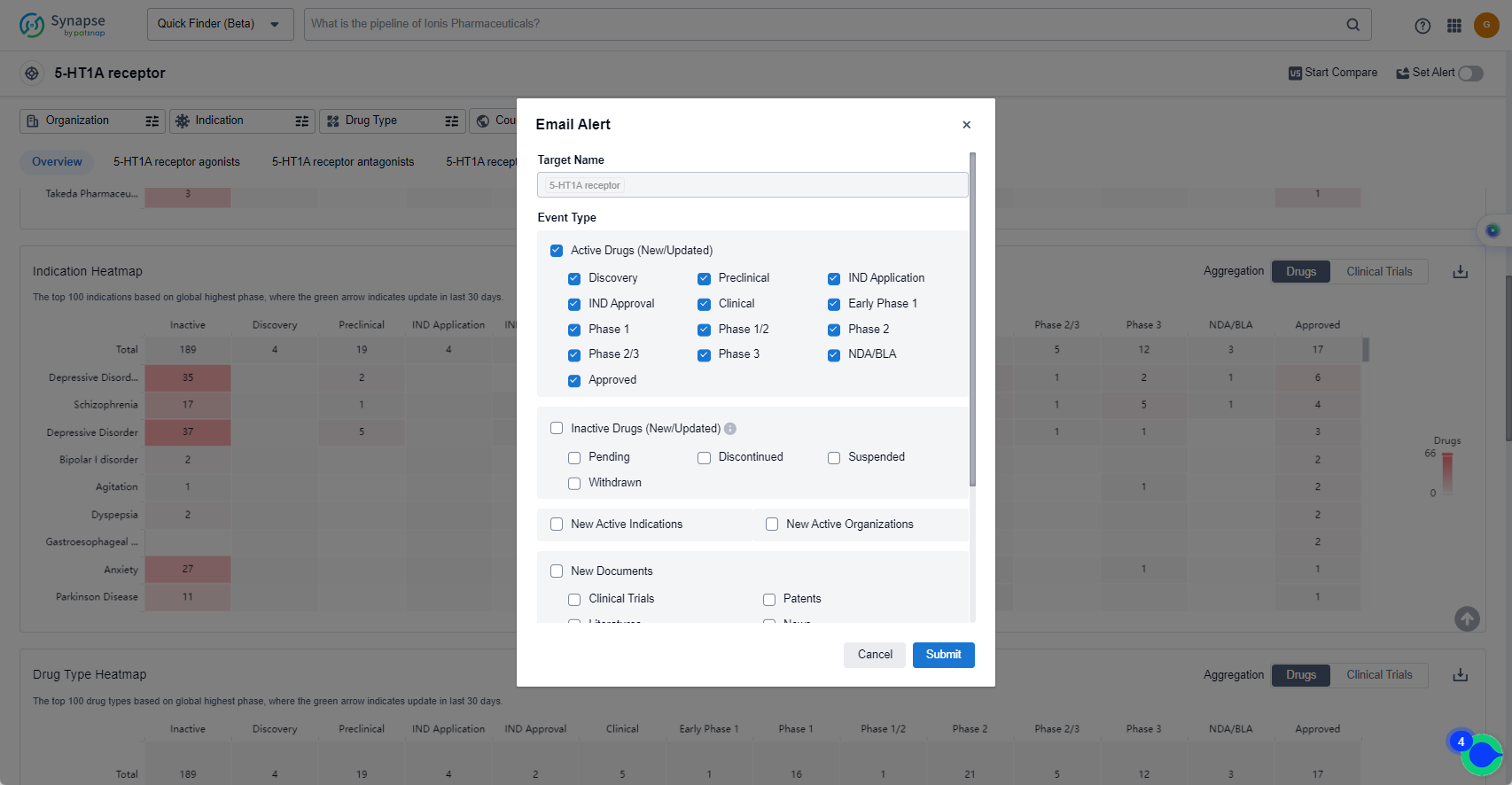
In the Synapse database, you can keep abreast of the latest research and development advances of 5-HT1A receptor agonists anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!