Longhorn Vaccine and Diagnostics' data shows lhnvd-201 can neutralize covid-19 and influenza coinfection
Longhorn Vaccines and Diagnostics, a One Health firm that focuses on making vaccines and diagnostic means for worldwide health and zoonosis issues, revealed fresh information from an animal experiment. The data illustrated that LHNVD-201, their uncombined peptide vaccine nominee, produced extensive and long-lasting neutralizing antibodies towards Covid-19 and influenza viruses.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
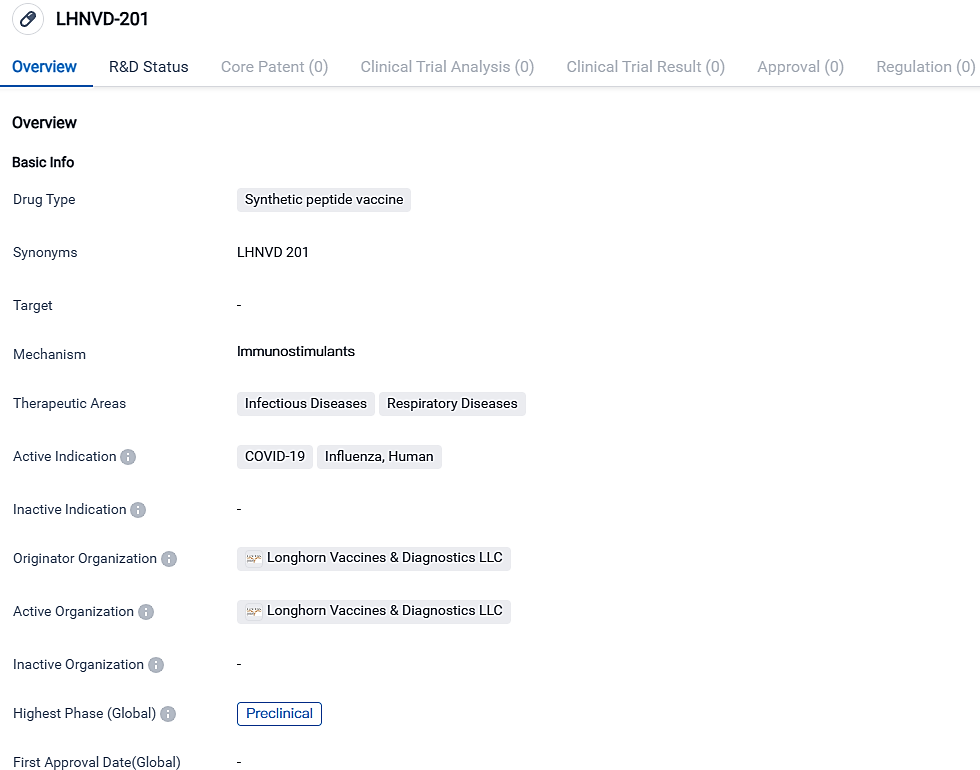
The research findings were showcased at the 17th Vaccine Congress held in Glasgow, Scotland from September 24 to 27, 2023. The new threat in the world after the pandemic is flu and Covid-19 seasonal infections along with mixed infection. For the purpose of evaluating co-vaccination as a plausible strategy for effective prevention, Longhorn conducted a study on the effectiveness of LHNVD-201 in mice, with a focus on its endurance and broad coverage of various strains. LHNVD-201 contains highly-conserved influenza neuraminidase and Matrix epitopes, as well as SARS-CoV-2 spike protein and RNA polymerase epitopes.
“Peptides that target conserved epitopes of different pathogens without being conferred can be a cost-effective and easily expandable vaccine approach for building comprehensive immunity against viruses such as Covid-19 and influenza," mentioned Gerald W. Fischer, MD, CEO of Longhorn Vaccines, and Diagnostics.
W. Fischer further added, “LHNVD-201, our peptide-based universal vaccine candidate, could produce neutralizing antibodies potently acting against various strains of influenza and COVID-19, hinting at the possibility of imparting longer-term immunity to these two major pathogens.”
In this analysis, Longhorn studied the mice that were immunized with 20µg of Covid-19 Pep05 and Covid-19 Pep11, combined with AddaVax™. The isotype-specific IgG levels towards Covid-19 and influenza peptides were assessed using an enzyme-linked immunosorbent assay, also recognized as ELISA.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
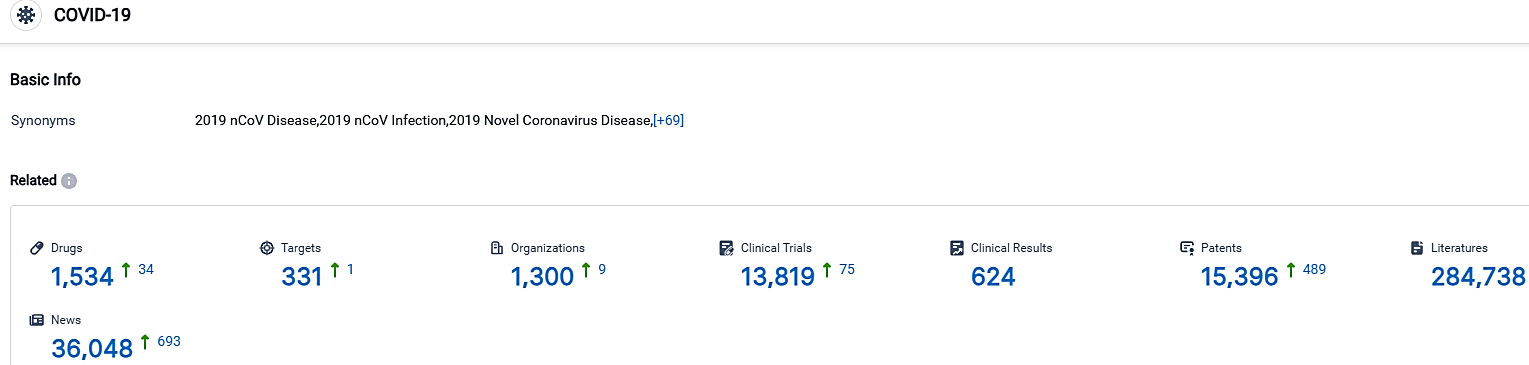
According to the data provided by the Synapse Database, As of September 30, 2023, there are 1534 investigational drugs for the COVID-19, including 331 targets,1300 R&D institutions involved, with related clinical trials reaching 13819,and as many as 15396 patents.
LHNVD-201 is being developed as a potential therapy for COVID-19 and Influenza in people. Still in its preclinical stage, the medication offers potential solutions in tackling the persistent worldwide health threats caused by these contagious respiratory ailments.