Proteasome Inhibitors Initiate a New Treatment Regime for Multiple Myeloma
Initially discovered in 1968, the proteasome is a complex enzyme located in the nuclei and cytoplasm of eukaryotic cells. The majority of proteasomes are 26s proteasomes, comprised of a 20s catalytic core and one or two 19s regulatory subunits at both ends. The 20s core structure is formed by stacking two α rings and two β rings together to form a barrel-shaped compound. Each β ring contains three protein degradation active sites: chymotrypsin-like (β5), trypsin-like (β2), and caspase-like (β1).
Proteasomes are a key component of the ubiquitin-proteasome pathway, a central platform for recycling proteins involved in crucial cellular processes, including cell cycle progression, DNA repair, and transcription. Proteins are timely degraded through the ubiquitin-proteasome pathway to maintain normal cellular homeostasis.
Multiple myeloma (MM) is a hematological malignancy characterized by the clonal proliferation of bone marrow plasma cells. Malignant plasma cells secrete more immunoglobulins than normal plasma cells, thus MM cells are highly dependent on the ubiquitin-proteasome pathway for survival. Therefore, proteasomes are logical targets for anti-MM therapy. Proteasome inhibitors work by blocking the degradation of associated proteins in the proteasome, leading to an accumulation of ubiquitinated and/or misfolded proteins in cells, thereby causing MM cell apoptosis.
Proteasome Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 22 Sep 2023, there are a total of 55 Proteasome drugs worldwide, from 71 organizations, covering 66 indications, and conducting 1637 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.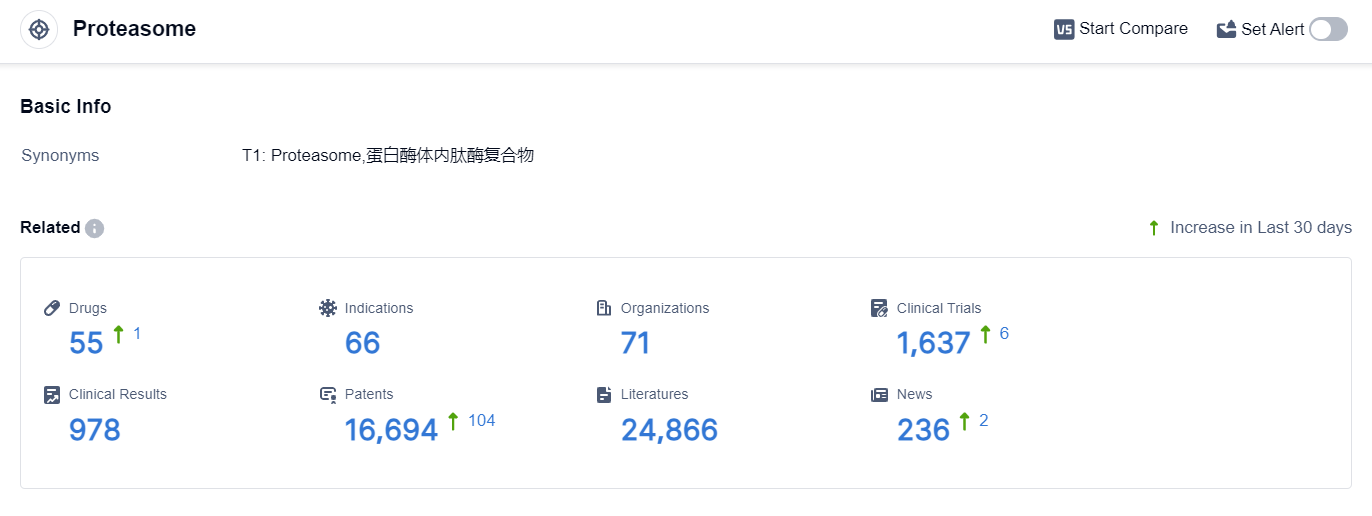
The target Proteasome in the pharmaceutical industry has seen significant growth and development. Takeda Pharmaceutical Co., Ltd. and Amgen, Inc. are the leading companies in terms of R&D progress, with drugs in multiple stages of development.
MM is the most approved indication for drugs targeting Proteasome. Small molecule drugs are the most rapidly progressing drug type, followed by Synthetic peptides, Chemical drugs, and Antibody drug conjugate (ADC).
The United States, China, and Japan are the countries with the fastest development under the target Proteasome. Overall, the competitive landscape for target Proteasome is dynamic, with multiple companies and countries actively involved in research and development. The future development of target Proteasome holds promise for the treatment of various indications and the advancement of innovative drug therapies.
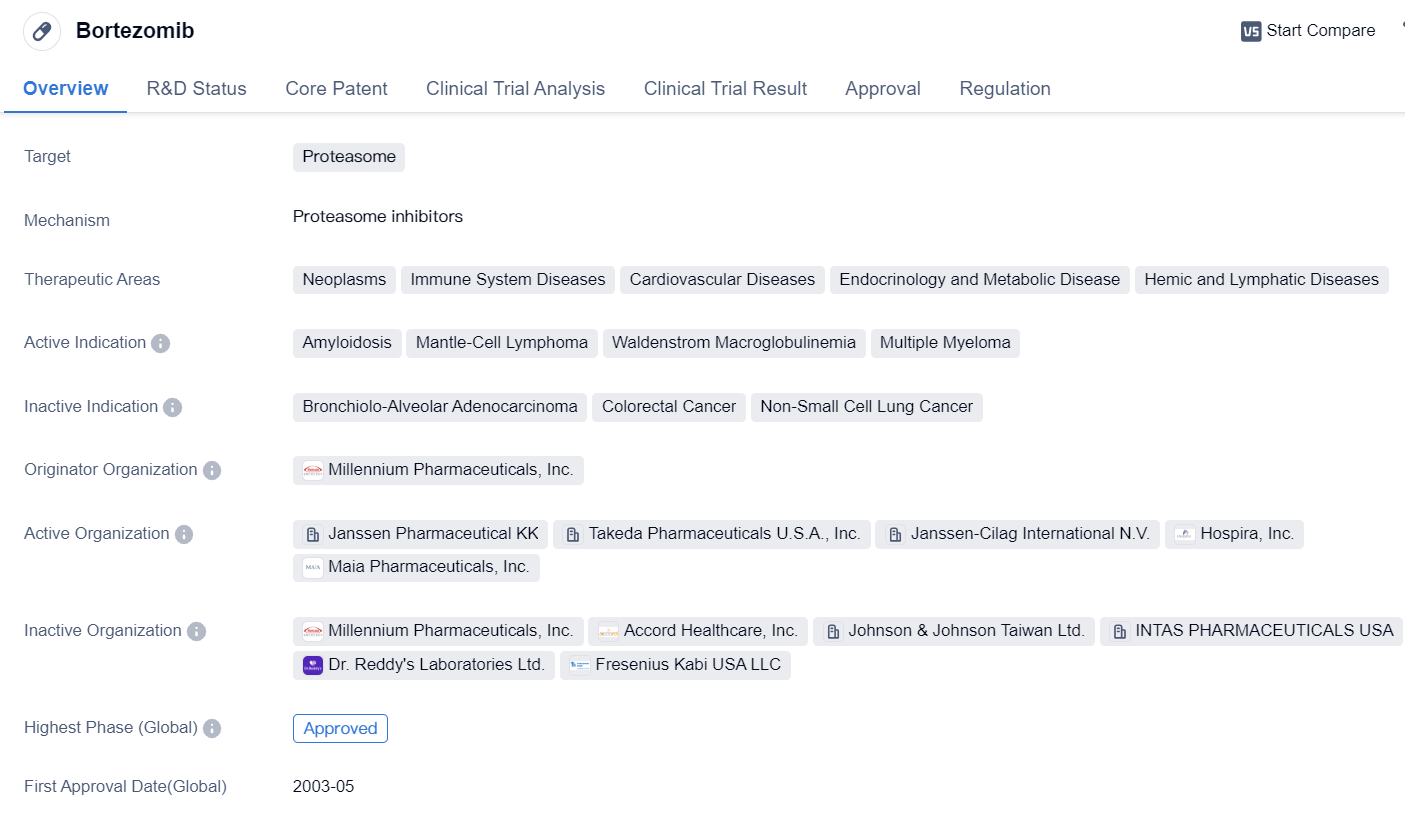
Key drug: Bortezomib
Bortezomib is a small molecule drug that belongs to the class of proteasome inhibitors. It is primarily used in the treatment of various neoplasms, immune system diseases, cardiovascular diseases, endocrinology and metabolic diseases, as well as hemic and lymphatic diseases. The drug has shown efficacy in treating amyloidosis, mantle-cell lymphoma, Waldenstrom macroglobulinemia, and MM.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization of Bortezomib is Millennium Pharmaceuticals, Inc. The drug has received approval for use in multiple countries, including the United States, where it was first approved in May 2003. Bortezomib has also obtained approval in China.
In terms of regulatory status, Bortezomib has been granted accelerated approval and orphan drug designation. Accelerated approval is a regulatory pathway that allows for the expedited approval of drugs that address unmet medical needs. Orphan drug designation is granted to drugs that are intended to treat rare diseases or conditions.
Bortezomib's mechanism of action involves inhibiting the proteasome, which is responsible for degrading proteins within cells. By inhibiting the proteasome, Bortezomib disrupts the normal protein degradation process, leading to the accumulation of proteins and ultimately inducing cell death.
The drug has shown promising results in clinical trials, leading to its approval for the treatment of various indications. Bortezomib has demonstrated efficacy in improving overall survival and progression-free survival in patients with MM, mantle-cell lymphoma, and Waldenstrom macroglobulinemia.
Overall, Bortezomib is a small molecule drug that targets the proteasome and has been approved for the treatment of multiple neoplasms and immune system diseases. Its approval in multiple countries, including the United States and China, highlights its global significance. The drug's regulatory status as an accelerated approval and orphan drug further emphasizes its potential in addressing unmet medical needs. Bortezomib's mechanism of action and clinical efficacy make it a valuable therapeutic option in the field of biomedicine.
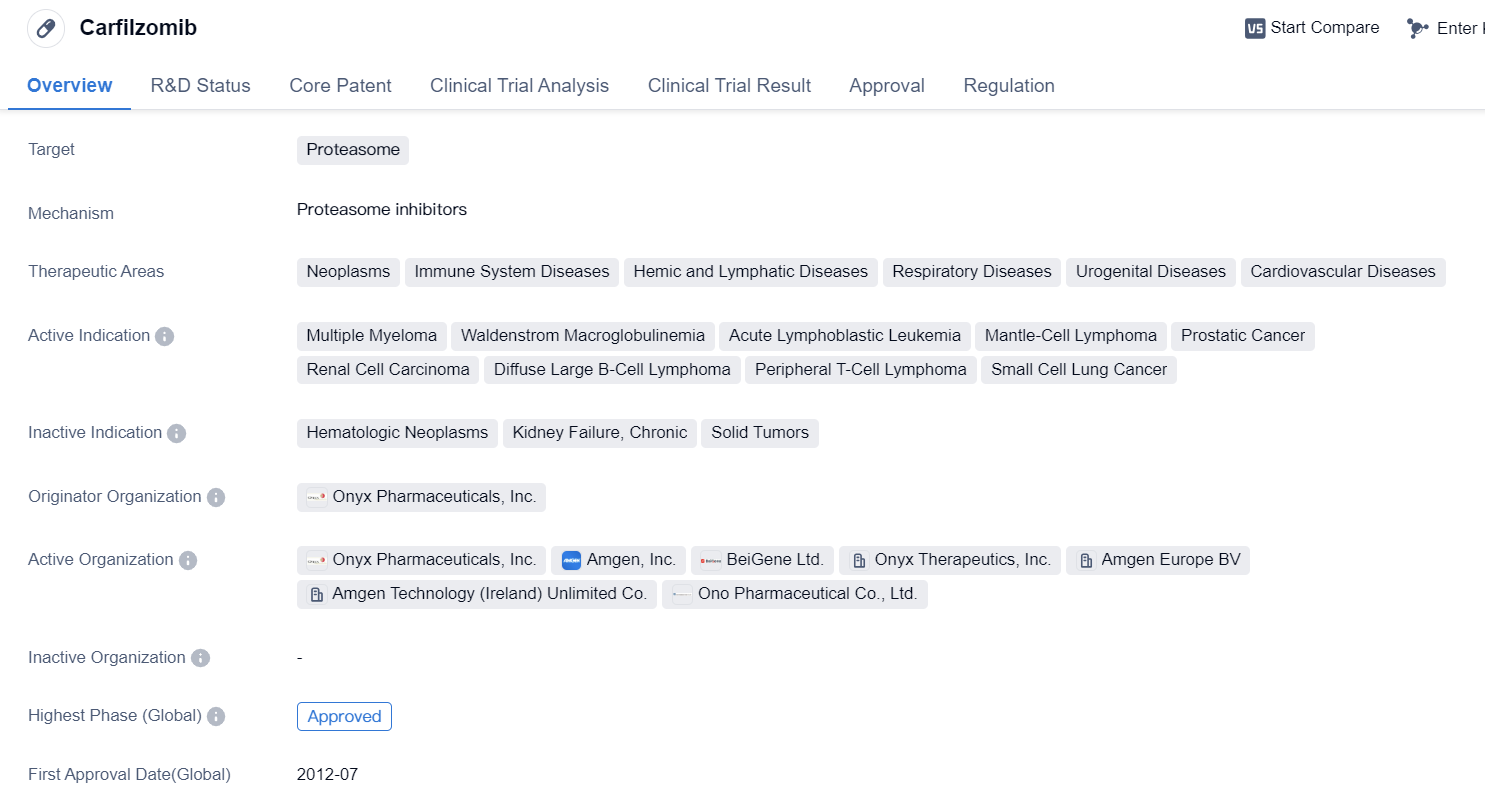
Carfilzomib
Carfilzomib is a synthetic peptide drug that targets the proteasome, which is a cellular complex responsible for degrading proteins. It has been approved for use in multiple therapeutic areas, including neoplasms (abnormal growth of cells), immune system diseases, hemic and lymphatic diseases, respiratory diseases, urogenital diseases, and cardiovascular diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown efficacy in treating various types of cancers, including MM, Waldenstrom macroglobulinemia, acute lymphoblastic leukemia, mantle-cell lymphoma, prostatic cancer, renal cell carcinoma, diffuse large B-cell lymphoma, peripheral T-cell lymphoma, and small cell lung cancer. This wide range of active indications highlights the potential of carfilzomib in combating different types of cancer.
Carfilzomib was developed by Onyx Pharmaceuticals, Inc., and it has received approval in both the global market and China. The drug obtained its first approval in the United States in July 2012, making it available for patients in need of treatment.
Regulatory agencies have recognized the importance of carfilzomib in addressing unmet medical needs, as evidenced by its designation as an accelerated approval, fast track, orphan drug, and special review project. These designations aim to expedite the development and approval process for drugs that show promise in treating rare diseases or conditions with limited treatment options.
In summary, carfilzomib is a synthetic peptide drug that targets the proteasome and has been approved for use in various therapeutic areas. It has shown efficacy in treating multiple types of cancer, and its approval in both the global market and China highlights its potential impact on a global scale. The drug was developed by Onyx Pharmaceuticals, Inc., and its regulatory designations reflect the recognition of its importance in addressing unmet medical needs.