Mabwell Reports Advancement in 9MW2821 Clinical Trials for Triple-Negative Breast Cancer
Mabwell, a biopharmaceutical firm noted for its innovation and comprehensive industry chain, disclosed developments in its clinical trial of the innovative Nectin-4-targeting ADC (9MW2821) designed for the treatment of triple-negative breast cancer.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In an ongoing single-agent clinical study involving 9MW2821, with a patient group of 20 individuals having locally advanced or metastatic triple-negative breast cancer and receiving a dose of 1.25 mg/kg, the outcomes for tumor evaluation revealed an objective response rate of 50% and a disease control rate of 80%. Among these patients, one achieved a complete response (CR) and has maintained CR status for 20 months and continuing.
The China National Medical Products Administration has approved the investigational new drug application for 9MW2821 in synergy with an immune checkpoint inhibitor for addressing triple-negative breast cancer.
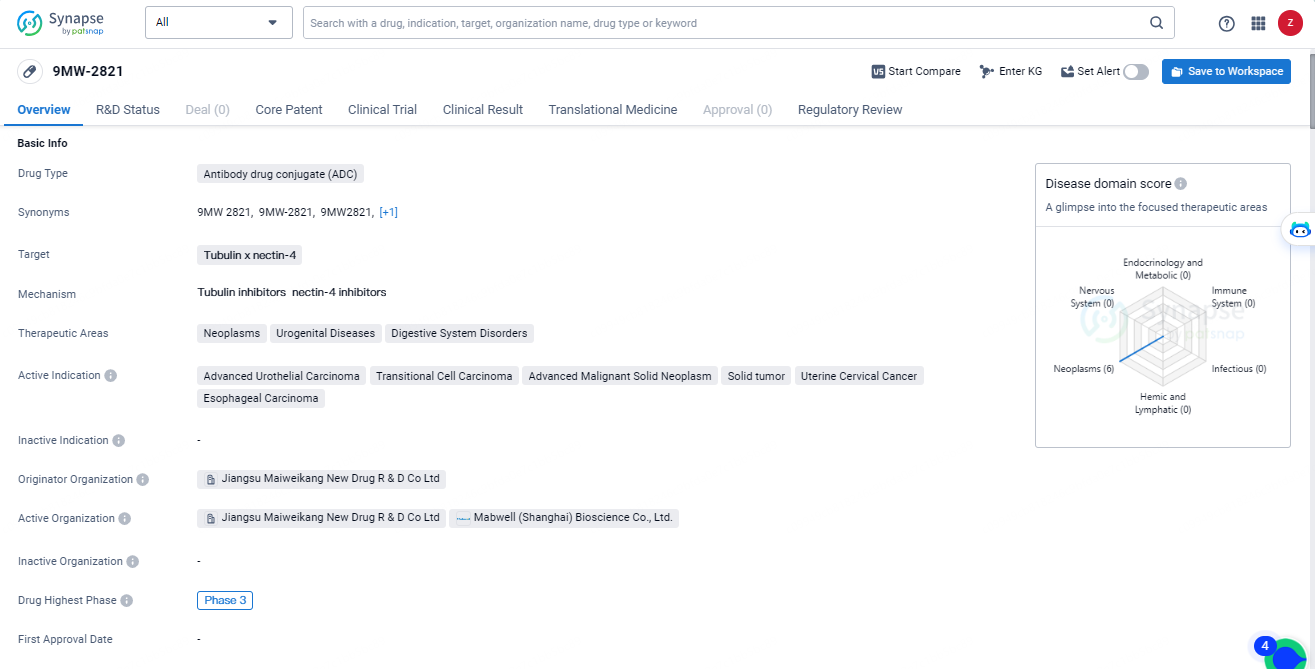
Currently, 9MW2821 is subject to numerous clinical trials across a range of indications. The Phase III study for monotherapy treating patients with locally advanced/metastatic urothelial carcinoma, who have previously received platinum-based chemotherapy and a PD-(L)1 inhibitor, has officially started; simultaneously, a Phase I/II trial exploring first-line combination therapy with a PD-1 inhibitor has also commenced. Both studies have successfully enrolled their first patients.
For cervical and esophageal cancers, Mabwell is pursuing approval for Phase III clinical trials and is additionally evaluating front-line combination therapy approaches, planning to file clinical trial applications soon. The U.S. Food and Drug Administration has awarded both Fast Track and Orphan Drug Designations to 9MW2821 for the treatment of advanced, recurrent, or metastatic esophageal squamous cell carcinoma, as well as esophageal cancer.
9MW2821 represents the pioneering site-specific conjugated Nectin-4-targeting ADC innovated by Mabwell through its ADC platform and an automated high-throughput hybridoma antibody molecular discovery platform. Moreover, it stands as the initial Nectin-4-targeting ADC from Chinese developers to enter clinical trials and is the first in the world to demonstrate clinical efficacy data against cervical, esophageal, and breast cancers.
In 2024, the U.S. FDA has conferred 9MW2821 with Fast Track and Orphan Drug Designations for managing advanced, recurrent, or metastatic esophageal squamous cell carcinoma in February and esophageal cancer in May respectively.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
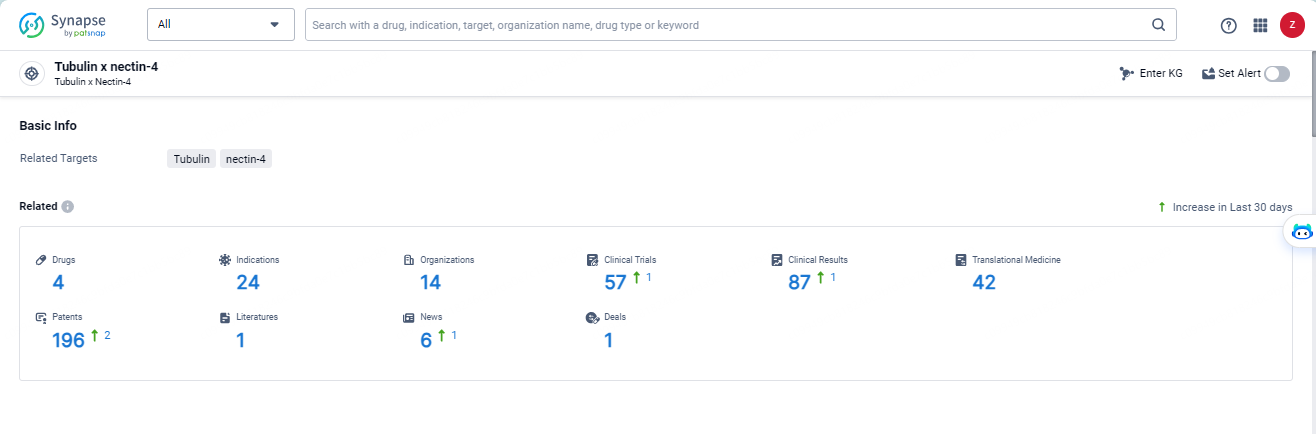
According to the data provided by the Synapse Database, As of May 14, 2024, there are 4 investigational drugs for the tubulin and nectin-4 target, including 24 indications, 14 R&D institutions involved, with related clinical trials reaching 57, and as many as 196 patents.
9MW-2821 is an ADC drug that targets tubulin and nectin-4, with a focus on treating neoplasms, urogenital diseases, and digestive system disorders. It is currently in Phase 3 of clinical development, indicating advanced stages of testing and potential efficacy. The drug has also received regulatory designations such as Fast Track and Orphan Drug, highlighting its potential to address unmet medical needs and rare diseases.