Tonix Pharmaceuticals Submits NDA for TNX-102 SL, Offering New Hope for Fibromyalgia Patients
On October 16, Tonix Pharmaceuticals announced that it has submitted a New Drug Application (NDA) to the U.S. FDA for TNX-102 SL (Cyclobenzaprine hydrochloride). This non-opioid, centrally acting analgesic is designed for the treatment of fibromyalgia. In two Phase 3 clinical trials, TNX-102 SL demonstrated significant pain relief and was generally well tolerated.

Fibromyalgia
Fibromyalgia is a common chronic pain disorder that affects individuals worldwide, with a prevalence rate of 2% to 4% in the general population. It does not present with visible clinical symptoms, and physical examinations only reveal increased sensitivity at specific "tender points" under pressure. Consequently, the number of people actually diagnosed with fibromyalgia is much lower. According to the classification criteria established by the American College of Rheumatology, diagnosis requires multiple tender points and chronic widespread pain. The disorder is characterized by the amplification of sensory and pain signals within the central nervous system, leading to chronic widespread pain, non-restorative sleep, fatigue, and cognitive dysfunction. Currently available treatment options are limited, and patients often express dissatisfaction with existing therapies.
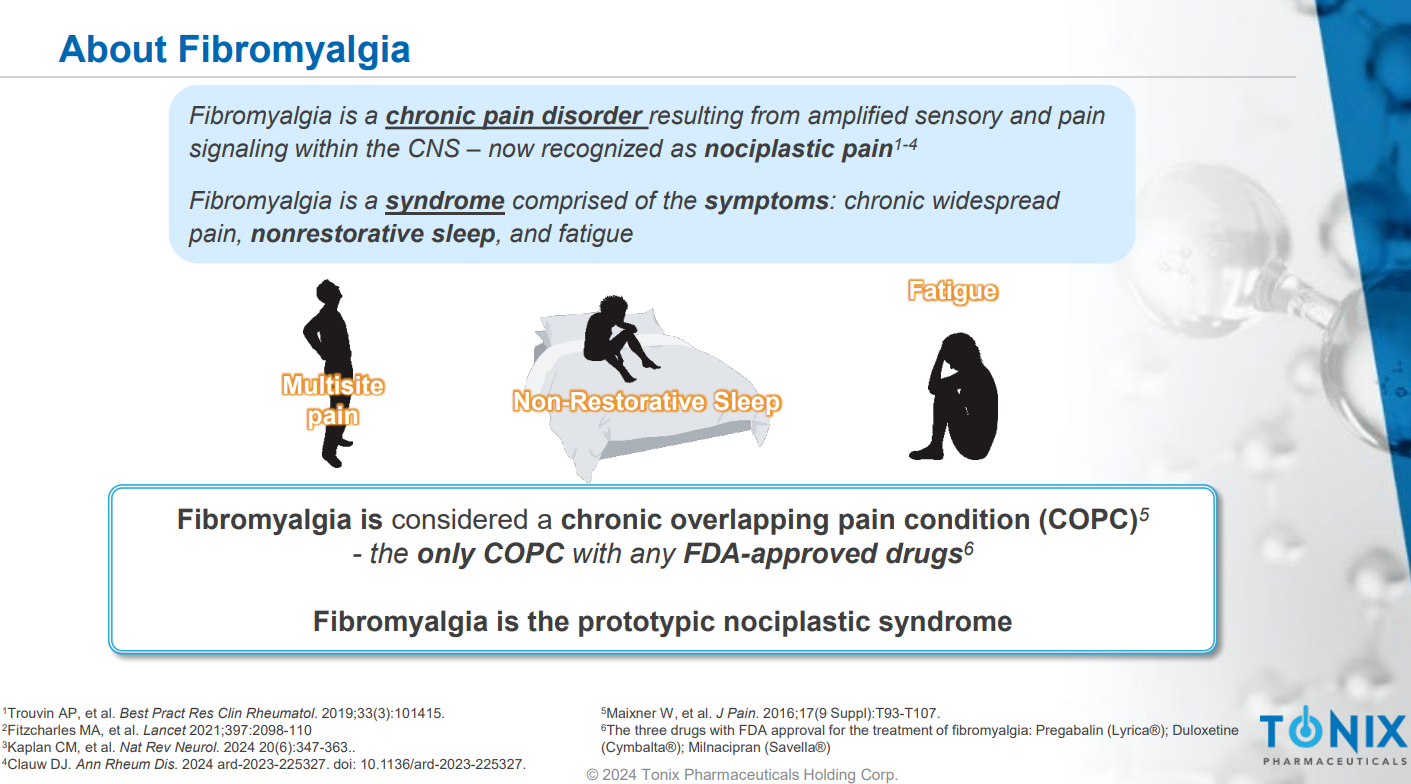
Fibromyalgia is complex, with symptoms divided into two groups: primary features, which include the most characteristic symptoms of fibromyalgia and are crucial according to the latest diagnostic criteria, and other common features.
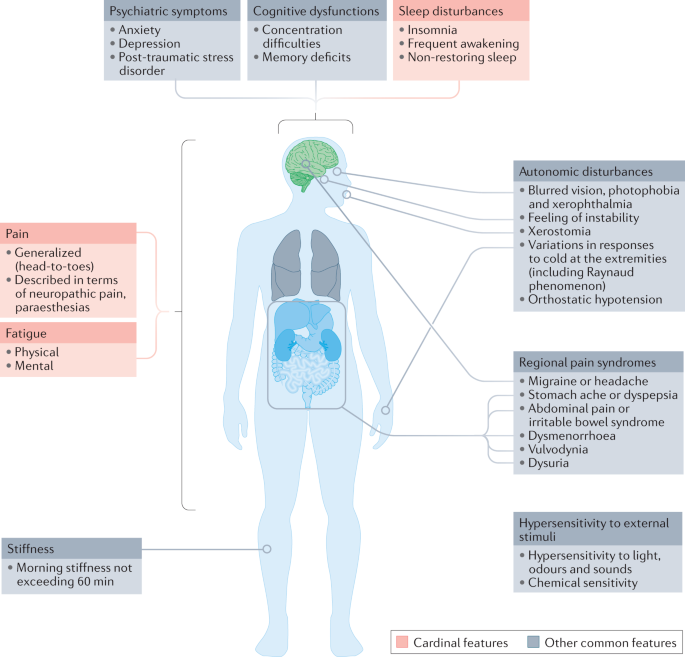
About TNX-102 SL
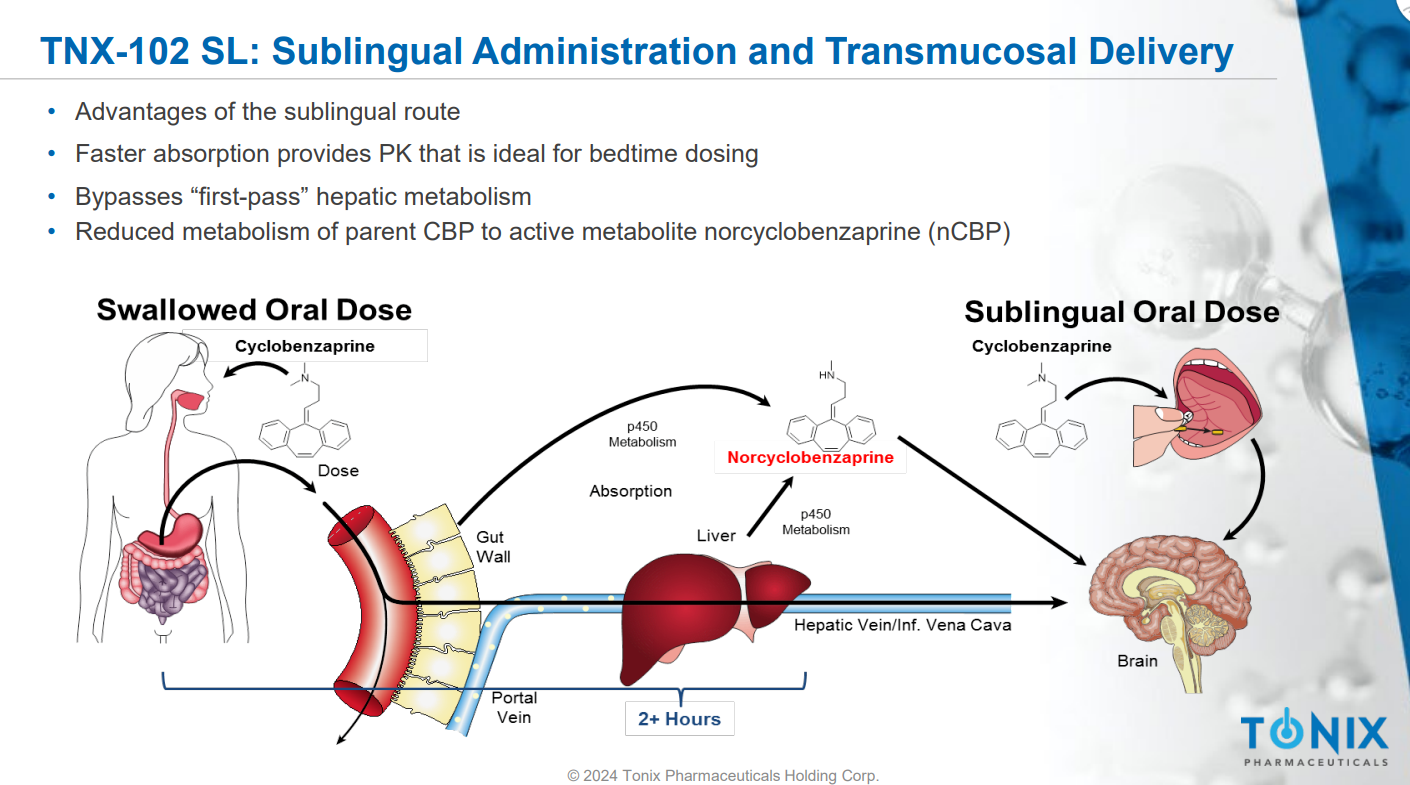
TNX-102 SL's primary ingredient is a sublingual formulation of Cyclobenzaprine. Its mechanism of action involves interacting with multiple GPCR-class central nervous system receptors, including 5-HT2A, α1-adrenergic, H1, and M1 muscarinic acetylcholine receptors. By engaging these receptors, TNX-102 SL aims to improve non-restorative sleep in fibromyalgia patients.
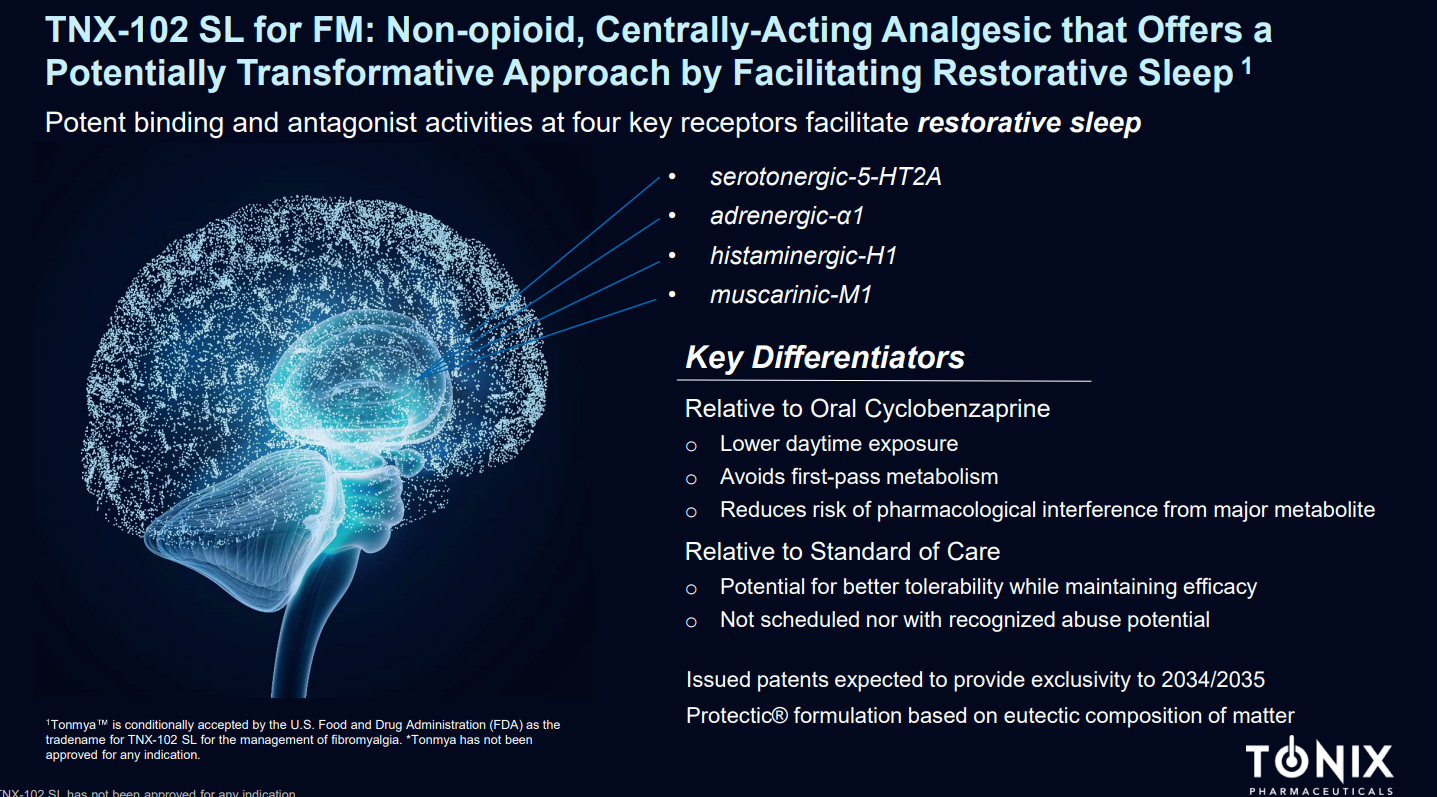
Administered sublingually, this medication is rapidly absorbed through the mucosa, bypassing the liver's first-pass metabolism and thereby reducing the production of long half-life active metabolites.
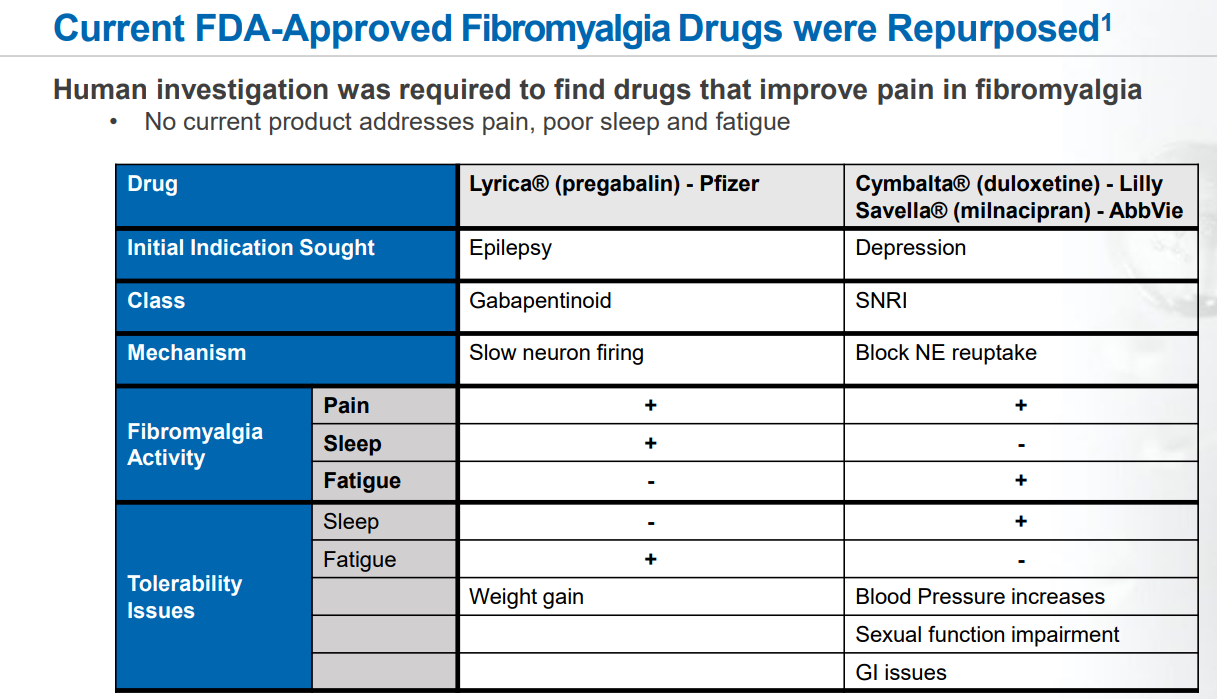
Currently, three FDA-approved drugs are available for fibromyalgia, representing two distinct drug classes. These include gabapentinoids, with Pfizer's Lyrica® (Pregabalin) approved in 2008, and SNRIs, represented by Eli Lilly's Cymbalta® (Duloxetine) and Abbvie's Savella® (Milnacipran), approved in 2007 and 2009, respectively. If approved by the FDA, TNX-102 SL would be the first new tricyclic medication for fibromyalgia treatment.
Clinical trial progress
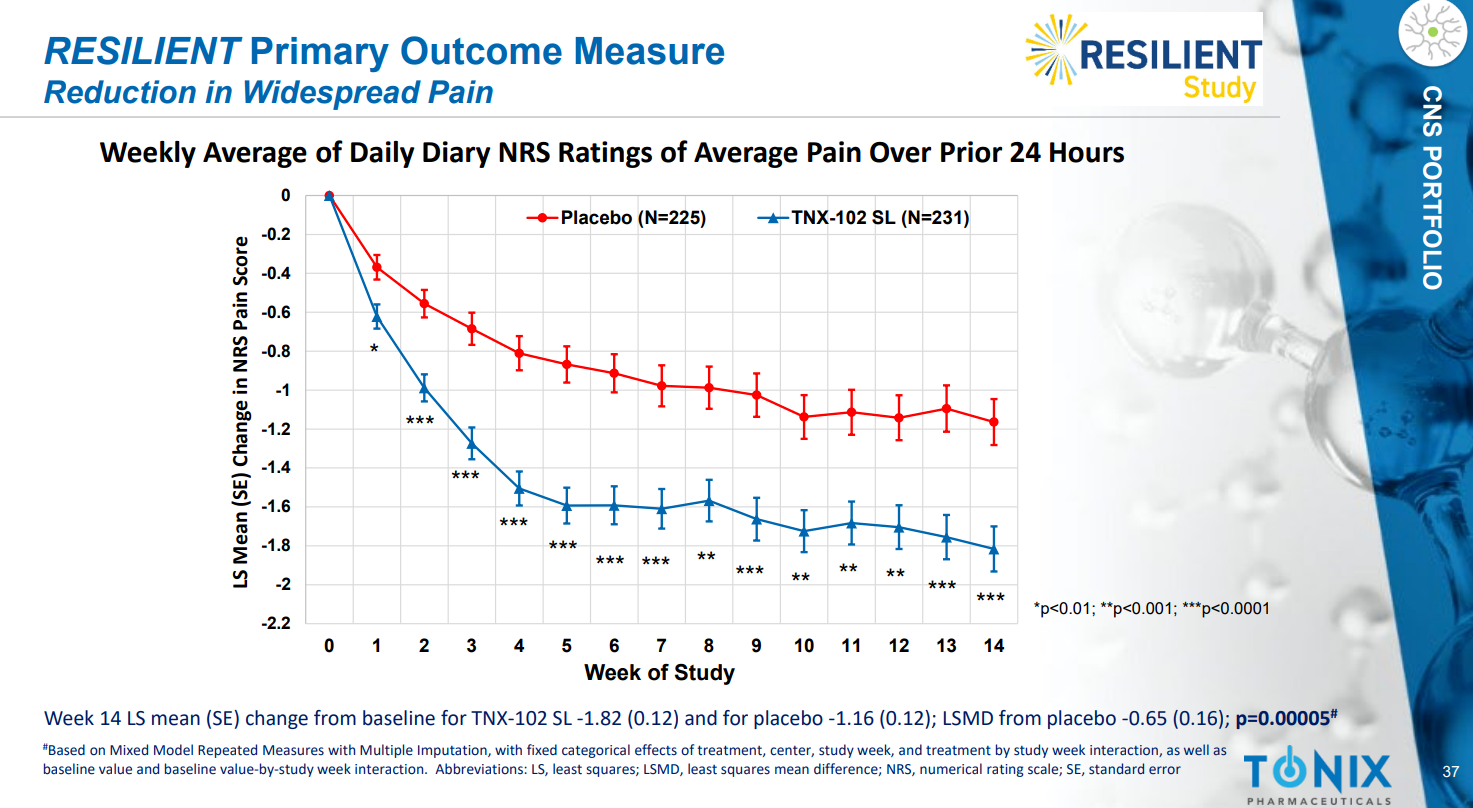
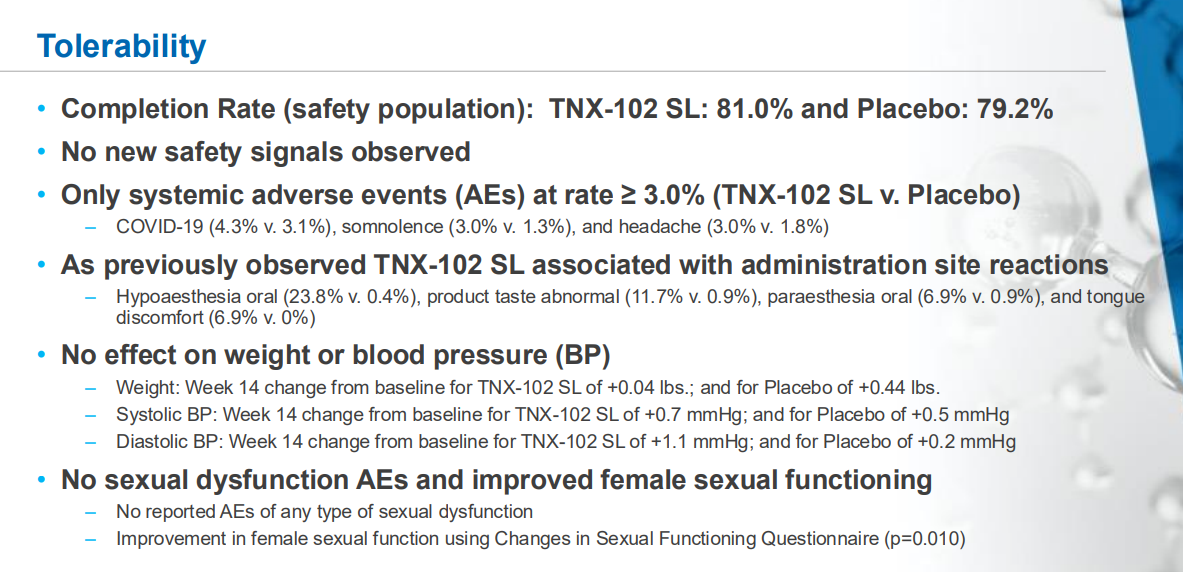
The NDA submission is backed by data from two 14-week double-blind, randomized, placebo-controlled Phase 3 clinical trials. Both studies showed that TNX-102 SL significantly reduced daily pain compared to placebo. The drug was generally well tolerated, with no new safety signals observed.

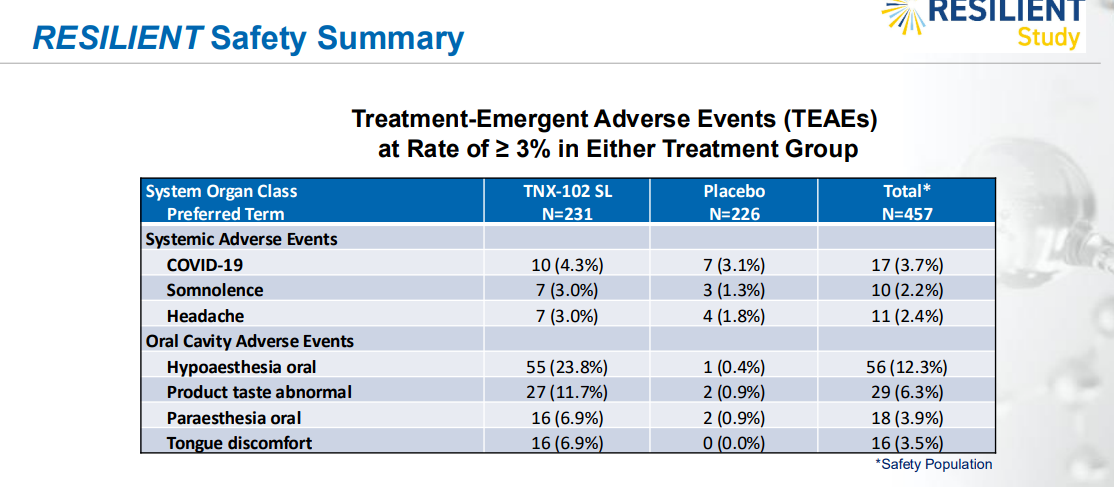
TNX-102 SL has received Fast Track designation from the FDA, aimed at accelerating the review of important new drugs that treat serious conditions and address unmet medical needs. The FDA typically has 60 days to assess whether the submitted NDA is complete and eligible for review. If accepted, an approval decision is expected in 2025.
"The submission of the NDA is a critical step forward in providing a new first-line treatment option for fibromyalgia patients," said Dr. Seth Lederman, CEO of Tonix. "TNX-102 SL will be the first member of a new drug class for treating fibromyalgia, and we believe it will offer patients a novel treatment choice."
"Despite the availability of three FDA-approved drugs, there is still an unmet need for new treatment options for fibromyalgia," commented Dr. Gregory Sullivan, Chief Medical Officer of Tonix. "If approved, TNX-102 SL will be the first new tricyclic medication for fibromyalgia treatment."
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!