Intellia Reports Positive Phase 2 Results for CRISPR Therapy NTLA-2002 in HAE Treatment
Intellia Therapeutics, Inc. (NASDAQ:NTLA), a prominent gene editing firm currently in the clinical stage, is dedicated to transforming healthcare through CRISPR-centered treatments. The company revealed encouraging Phase 2 results from its ongoing Phase 1/2 trial of NTLA-2002 in individuals with hereditary angioedema (HAE). The findings continue to support the potential of NTLA-2002 to fully prevent HAE episodes after a single infusion. This investigational therapy employs in vivo CRISPR-based gene editing and is being developed as a one-time intervention for HAE, a rare hereditary disorder that can result in severe and potentially life-threatening swelling episodes. The results were made publicly available today in The New England Journal of Medicine and will be shared on Saturday, October 26, during the 2024 American College of Allergy, Asthma & Immunology (ACAAI) Scientific Meeting in Boston, Massachusetts.
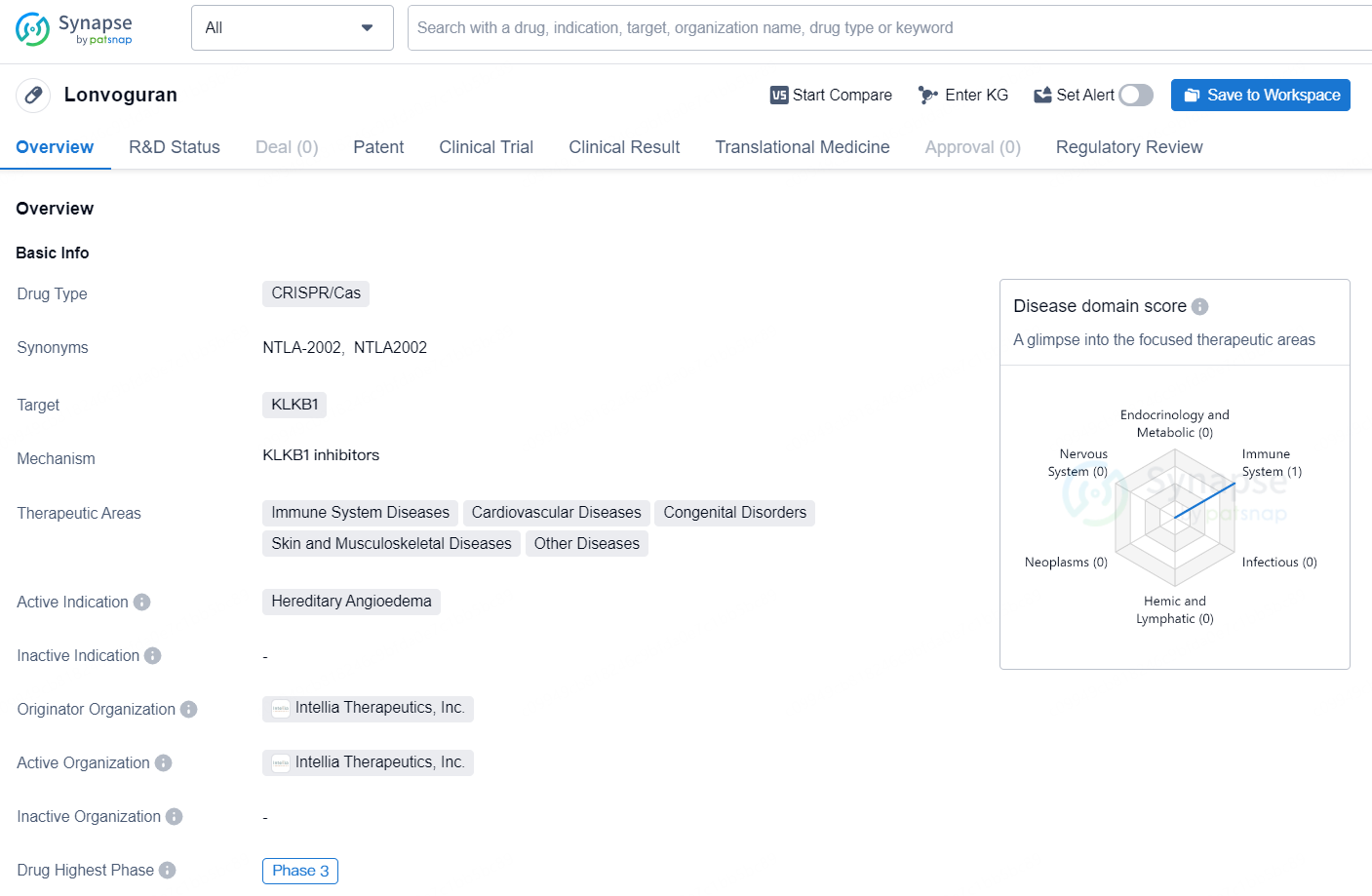
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Intellia President and CEO John Leonard, M.D., highlighted the promising outcomes of the NTLA-2002 Phase 2 trial, stating, “These results emphasize the significant potential of our in vivo CRISPR gene editing therapy to serve as a functional cure and transform the treatment approach for HAE. The Phase 2 findings revealed that a significant portion of patients in the 50 mg group achieved a complete response—experiencing no attacks and requiring no further treatment—following a single infusion of NTLA-2002, aligning with long-term Phase 1 results. We are very encouraged by these findings, which we believe distinguish NTLA-2002 from existing prophylactic therapies. What once seemed an extraordinary possibility for freedom from chronic treatment is now one step closer to realization for the HAE community.”
Dr. Danny Cohn, Ph.D., an internist from the Department of Vascular Medicine at Amsterdam University Medical Center and lead investigator of the Phase 2 trial, commented, “Current HAE therapies can mitigate but often do not fully eliminate angioedema episodes and necessitate ongoing administration, leading to a considerable treatment burden and negatively affecting the quality of life for HAE patients. The results from the NTLA-2002 Phase 2 trial are remarkable, indicating that this experimental therapy may permanently halt swelling episodes following a single infusion. I am hopeful that NTLA-2002 will revolutionize HAE treatment and eliminate the need for lifelong chronic therapies.”
The Phase 2 clinical trial was a randomized, double-blind, placebo-controlled study designed to assess the efficacy, safety, pharmacodynamics, and pharmacokinetics of NTLA-2002. A total of 27 subjects were enrolled and randomly assigned to receive one of two doses of NTLA-2002 (25 mg or 50 mg) or a placebo via intravenous infusion. The cutoff date for data analysis was April 4, 2024, after the 25th participant completed the 16-week primary observation period.
The administration of a single dose of NTLA-2002 resulted in substantial reductions in attack rates during the primary observation period. The mean monthly attack rates decreased by 75% and 77% for the 25 mg and 50 mg groups, respectively, compared to placebo during weeks 1-16, and by 80% and 81% during weeks 5-16. In the 50 mg group, eight out of 11 patients achieved a complete response with no attacks during the 16-week observation time; these patients remained attack-free through the latest follow-up (median of eight months), with no further treatment necessary. In contrast, four out of ten patients in the 25 mg group and none in the placebo group achieved a complete response. Additionally, those receiving the 50 mg dose experienced a greater reduction in kallikrein protein levels, with an average decrease of 86% from baseline compared to 55% in the 25 mg group at week 16.
Both dosage levels of NTLA-2002 were well tolerated. The most commonly reported adverse events included headache, fatigue, and nasopharyngitis. There were no severe adverse events, and all reported events were classified as Grade 1 or 2, except for one patient in the placebo cohort who experienced a serious Grade 4 adverse event of tongue edema with breathing difficulties deemed related to their existing HAE condition. No significant laboratory abnormalities were noted.
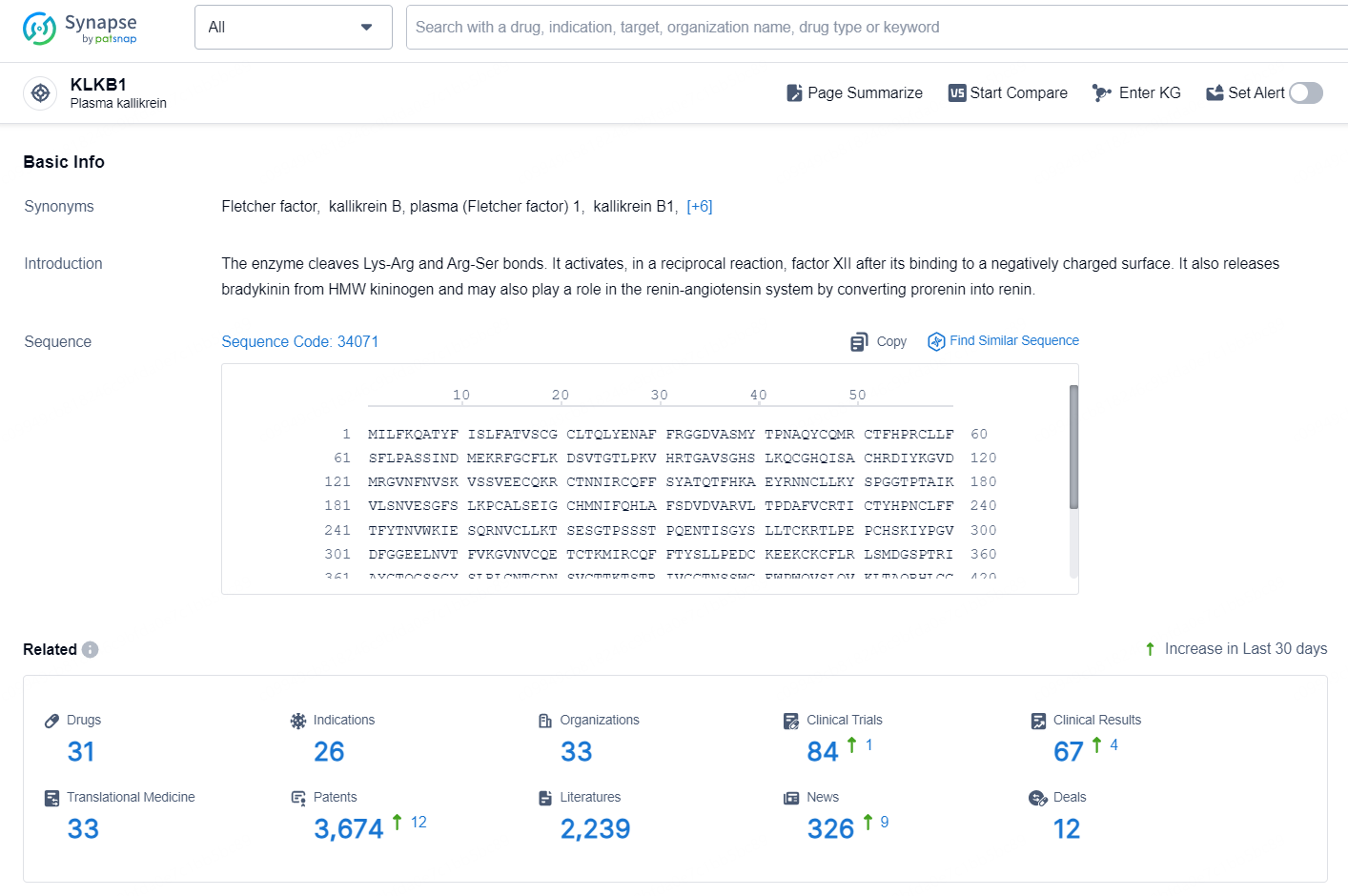
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 29, 2024, there are 31 investigational drugs for the KLKB1 target, including 26 indications, 33 R&D institutions involved, with related clinical trials reaching 84, and as many as 3674 patents.
"Lonoctocog alfa" is a CRISPR/Cas drug that targets KLKB1 and is developed by Intellia Therapeutics, Inc. It is currently in the highest global phase, which is Phase 3, for the treatment of Hereditary Angioedema. The drug falls under the therapeutic areas of Immune System Diseases, Cardiovascular Diseases, Congenital Disorders, Skin and Musculoskeletal Diseases, and Other Diseases.