Medications intervening from the root of difficult and complex diseases: Oligonucleotides
Oligonucleotides (ONs) are a class of drugs composed of single or double strands of oligonucleotides synthesized by artificial chemistry. They operate on mRNA through base complementary pairing, interfering with gene unwinding, replication, transcription, mRNA splicing and processing, output, translation and other stages. They cause abnormally encoded genes to lose function, thereby preventing the expression of "wrong" proteins, and thus exert a unique regulatory mechanism at the gene level on the transcription and translation process of disease genes.
Due to the unique chemical structure of oligonucleotide drugs, they exhibit poor drug properties: large molecular weight, strong hydrophilicity, high negative charge, not following Lipinski’s rule. Also, their pharmacokinetic characteristics are poor, they cannot pass through biological membranes, and there might be off-target effects. With the FDA's approval of Mipomersen for the treatment of Homozygous Familial Hypercholesterolemia (HoFH) in 2013 (now discontinued), it was verified that oligonucleotide drugs can be used outside of ophthalmology, which also opened the gene therapy era based on oligonucleotide drugs and brought great opportunities for the development of gene therapy.
Some rare and difficult diseases, such as Duchenne Muscular Dystrophy, Hepatitis B and peripheral neuropathy caused by hATTR Amyloidosis, cannot be effectively cured by traditional treatments. However, oligonucleotide drugs can interfere from the root to achieve both symptomatic and radical treatment, increasing medication choices for patients. Compared with traditional protein-targeted drugs, oligonucleotide drugs are more specific and efficient and are expected to fill the treatment field of protein-targeted drugs. With the advancement of technology, the indications have extended to chronic disease treatment, for example, LEQVIO, an siRNA approved by the EMA in 2020 for lipid reduction, requires only two injections per year.
In recent years, the continuous development of gene therapy drugs has brought new medication choices for cancer and rare disease patients. The research on oligonucleotide drugs has been going on for more than 30 years. Currently, 15 types of oligonucleotide drugs have been approved for marketing, but there are still issues, such as the modification of the structure of oligonucleotide drugs, optimization of pharmacokinetic characteristics, improvement of delivery systems, enhancement of efficacy, and reduction of side effects, which need continuous exploration.
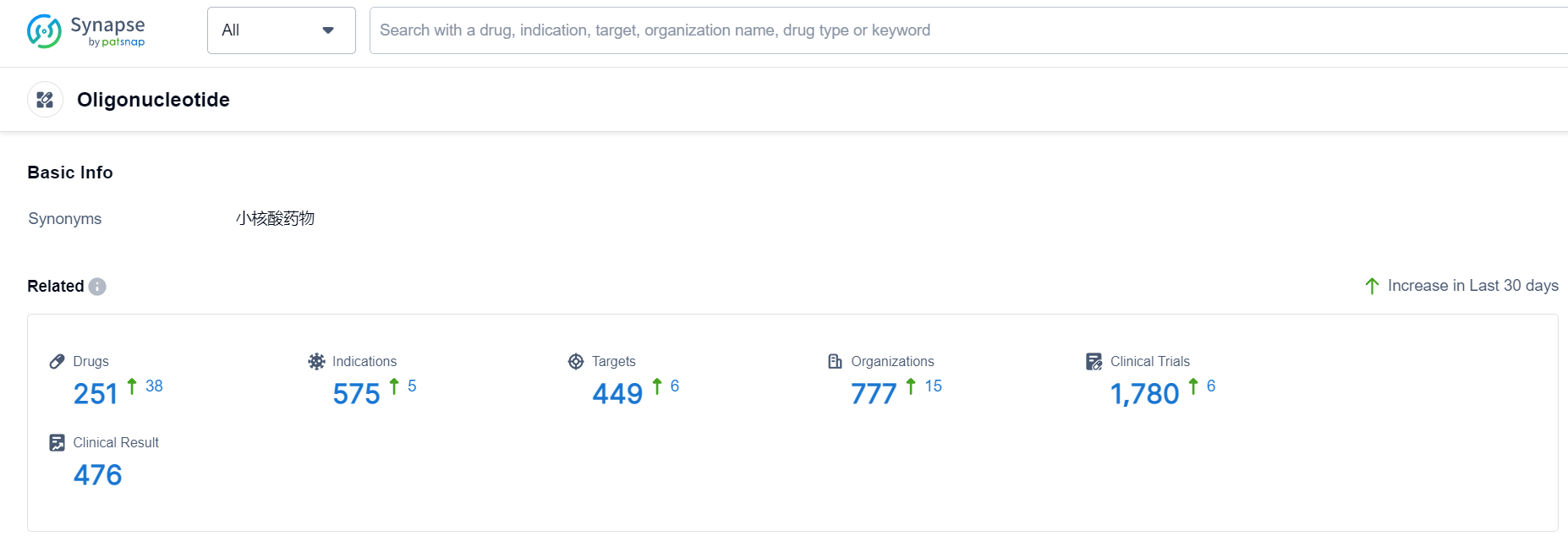
Oligonucleotide Competitive Landscape
According to Patsnap Synapse, as of 19 Sep 2023, there are a total of 251 Oligonucleotide drugs worldwide, from 777 organizations, covering 449 targets, 575 indications, and conducting 1780 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this drug type.
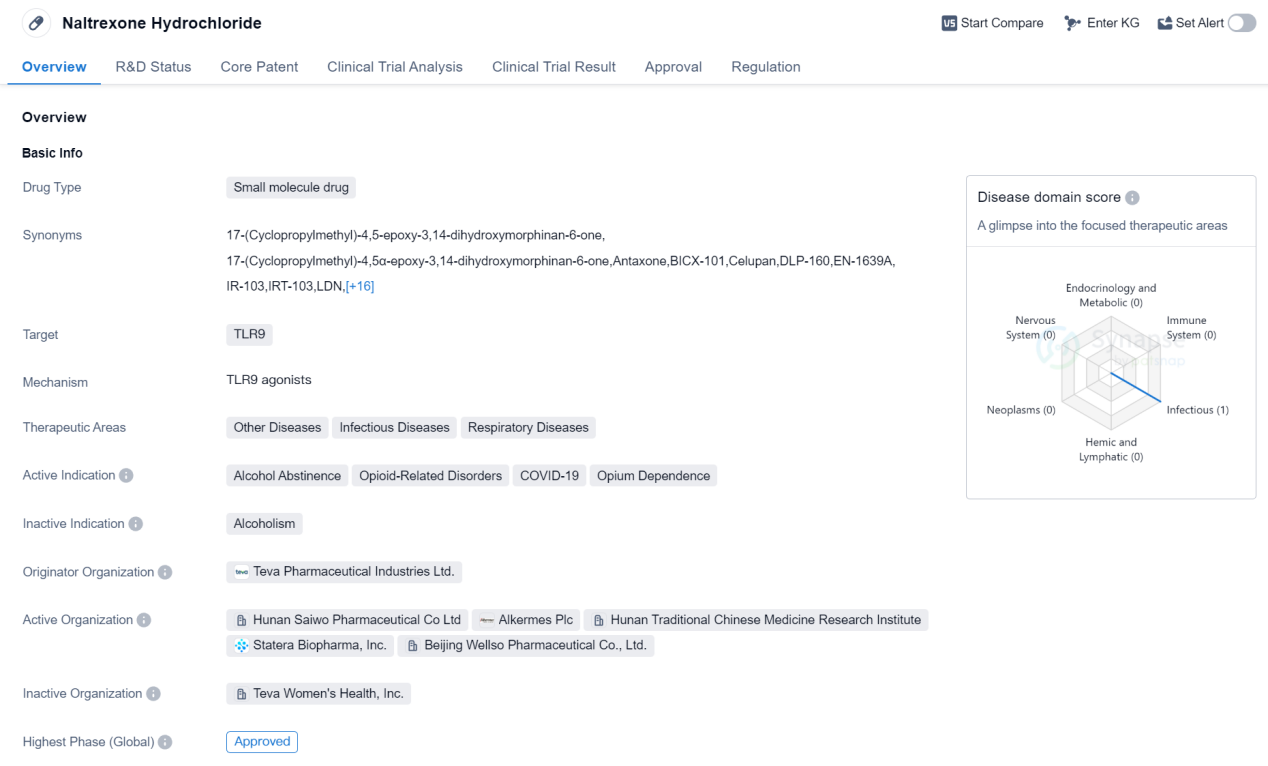
Approved Oligonucleotide Medicinal: Naltrexone Hydrochloride
Naltrexone Hydrochloride is a small molecule drug that targets Toll-like receptor 9 (TLR9). It is primarily used in the treatment of alcohol abstinence, opioid-related disorders, opium dependence, and has also shown potential in the management of COVID-19. The drug was first approved in the United States in November 1984 and is currently approved in both the global and Chinese markets.
Naltrexone Hydrochloride is developed by Teva Pharmaceutical Industries Ltd., a prominent player in the pharmaceutical industry. The drug has undergone rigorous testing and has successfully reached the highest phase of development, receiving approval for its therapeutic use. It is classified as an orphan drug, indicating its potential to treat rare diseases or conditions.
The therapeutic areas of Naltrexone Hydrochloride extend beyond its primary indications. It has shown promise in the treatment of other diseases, infectious diseases, and respiratory diseases. This versatility highlights the drug's potential to address a wide range of medical conditions.
The approval of Naltrexone Hydrochloride in 1984 marked a significant milestone in the pharmaceutical industry. Since then, it has gained recognition for its effectiveness in promoting alcohol abstinence and managing opioid-related disorders. The drug's approval in China further expands its reach and potential impact on a global scale.
Naltrexone Hydrochloride has been granted priority review status, indicating its importance in addressing unmet medical needs. This regulatory designation expedites the drug's review process, allowing it to reach patients in need more quickly.
In summary, Naltrexone Hydrochloride is a small molecule drug developed by Teva Pharmaceutical Industries Ltd. It targets TLR9 and is primarily used in the treatment of alcohol abstinence, opioid-related disorders, opium dependence, and has potential applications in COVID-19 management. The drug has received approval in the global markets, with its first approval dating back to 1984 in the United States. Its therapeutic areas extend beyond its primary indications, and it has been granted priority review status, emphasizing its significance in addressing medical needs.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
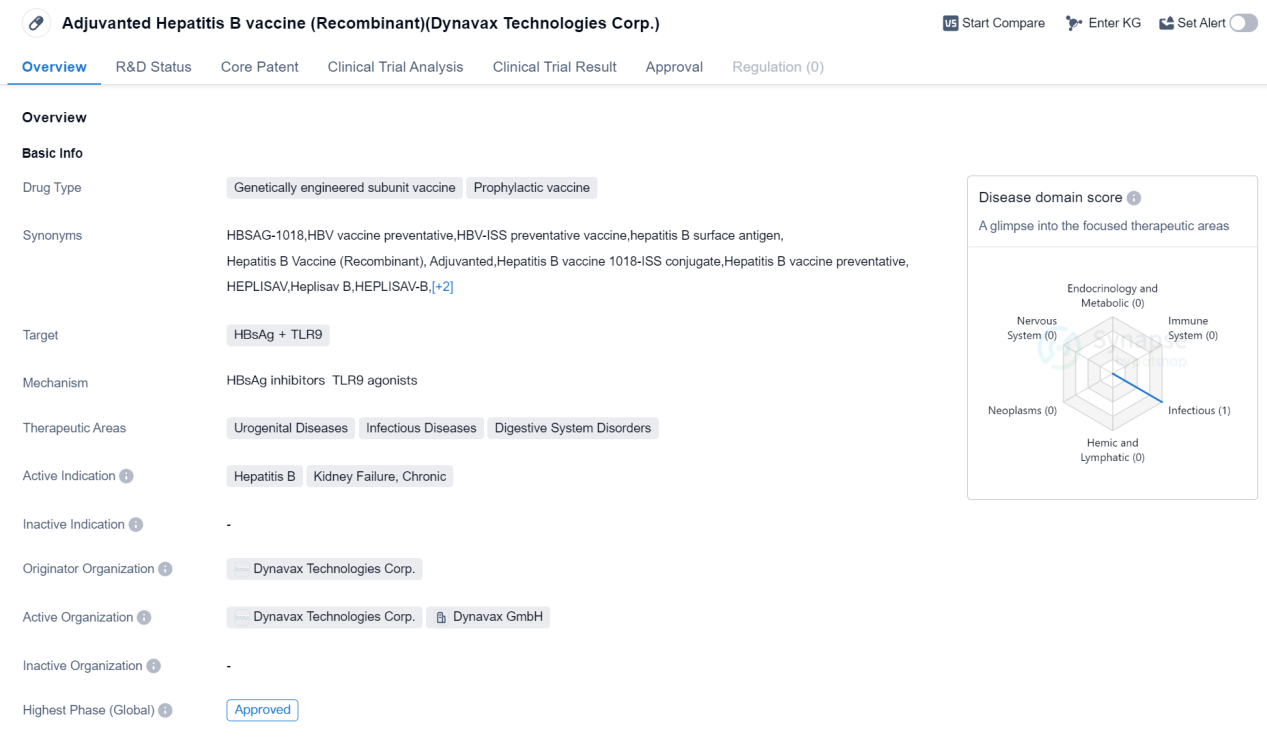
Approved Oligonucleotide Medicinal: Adjuvanted Hepatitis B vaccine
The Adjuvanted Hepatitis B vaccine is a genetically engineered subunit vaccine that falls under the category of prophylactic vaccines. It is designed to target the HBsAg (Hepatitis B surface antigen) and TLR9 (Toll-like receptor 9). This vaccine is primarily used for the prevention of Hepatitis B, a viral infection that affects the liver. Additionally, it is also indicated for the treatment of kidney failure, chronic.
The therapeutic areas in which this vaccine is applicable include urogenital diseases, infectious diseases, and digestive system disorders. Urogenital diseases encompass conditions related to the urinary and reproductive systems, while infectious diseases refer to illnesses caused by pathogenic microorganisms. Digestive system disorders involve ailments affecting the gastrointestinal tract.
The Adjuvanted Hepatitis B vaccine (Recombinant) is developed by Dynavax Technologies Corp., a pharmaceutical company specializing in the field of biomedicine. As of the highest phase, this vaccine has received approval, indicating that it has successfully undergone rigorous testing and evaluation to ensure its safety and efficacy.
The first approval of this vaccine took place in November 2017 in the United States. This implies that it has met the regulatory requirements set by the U.S. Food and Drug Administration (FDA) for commercial distribution and use within the country.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, the Adjuvanted Hepatitis B vaccine (Recombinant) is a genetically engineered subunit vaccine developed by Dynavax Technologies Corp. It is primarily used for the prevention of Hepatitis B and is also indicated for the treatment of kidney failure, chronic. This vaccine targets the HBsAg and TLR9 and falls under the therapeutic areas of urogenital diseases, infectious diseases, and digestive system disorders. Having received approval, it is considered a safe and effective option for immunization against Hepatitis B. The first approval of this vaccine occurred in November 2017 in the United States.