Methyclothiazide: Detailed Review of its Transformative R&D Success, Mechanism of Action, and Drug Target
Methyclothiazide's R&D Progress
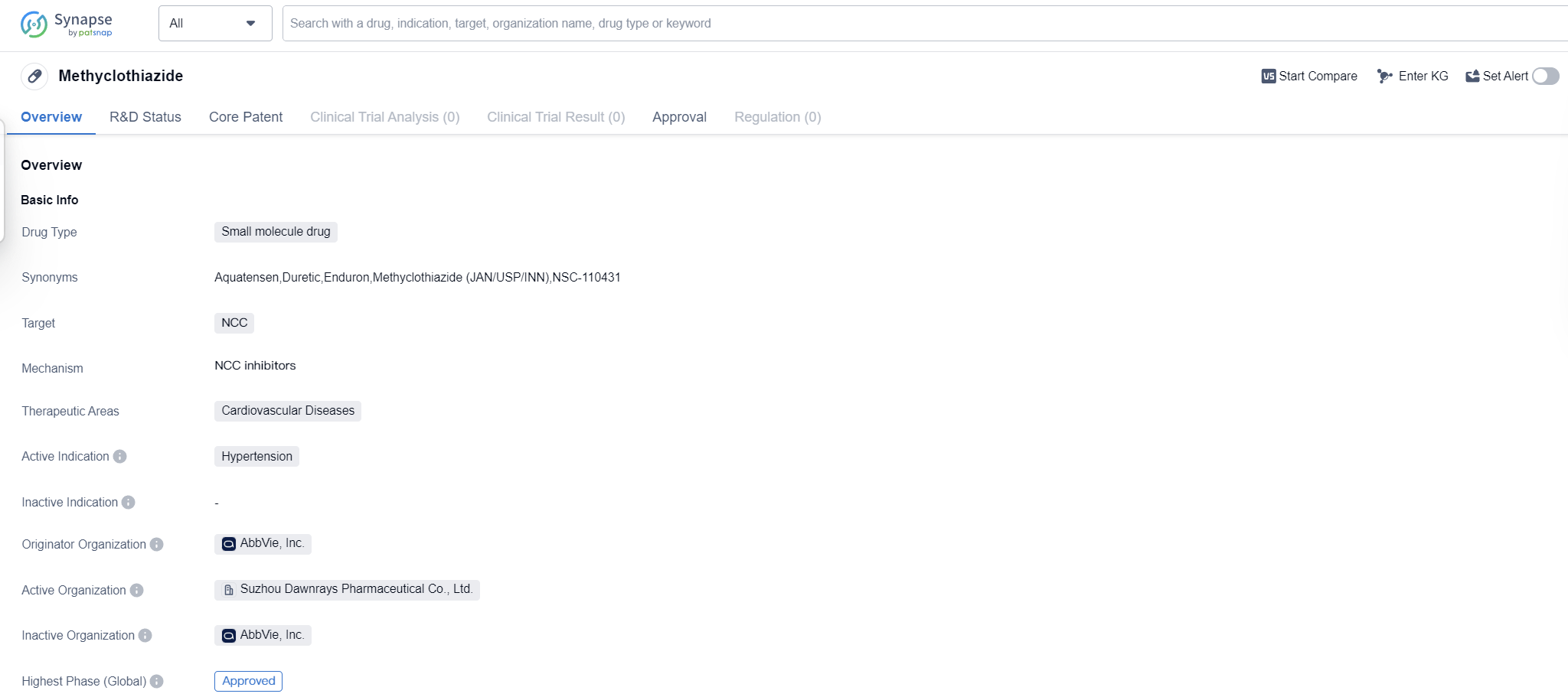
Methyclothiazide is a small molecule drug that falls under the therapeutic area of cardiovascular diseases. It is primarily used for the treatment of hypertension. The drug targets the NCC (sodium-chloride cotransporter) in the body.
Methyclothiazide was first approved for use in the United States in October 1960, making it a well-established medication in the field of biomedicine. The drug is developed and marketed by AbbVie, Inc., a renowned pharmaceutical organization.
Methyclothiazide has reached the highest phase of development which is approved globally. This suggests that the drug has undergone rigorous testing and clinical trials to demonstrate its therapeutic benefits and minimal side effects.
As a small molecule drug, Methyclothiazide is designed to interact with specific molecular targets in the body, in this case, the NCC. By targeting this transporter, the drug helps to regulate the balance of sodium and chloride ions in the body, ultimately reducing blood pressure and managing hypertension.
The approval of Methyclothiazide in 1960 highlights its long-standing presence in the pharmaceutical market. Over the years, it has likely gained recognition and trust from healthcare professionals for its efficacy in treating hypertension.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for methyclothiazide: NCC inhibitors
NCC inhibitors are a type of medication that specifically target and inhibit the activity of the sodium-chloride cotransporter (NCC). The NCC is a protein found in the kidneys that plays a crucial role in the reabsorption of sodium and chloride ions from the urine back into the bloodstream. By inhibiting the NCC, these inhibitors reduce the reabsorption of sodium and chloride, leading to increased excretion of these ions in the urine.
From a biomedical perspective, NCC inhibitors are primarily used in the treatment of conditions such as hypertension (high blood pressure) and edema (fluid retention). By blocking the NCC, these inhibitors help to reduce the overall volume of fluid in the body, which in turn decreases blood pressure and relieves swelling caused by excess fluid accumulation.
NCC inhibitors can be classified into different drug types, such as thiazide-like diuretics, which include medications like hydrochlorothiazide and chlorthalidone. These drugs act as NCC inhibitors by binding to the NCC protein and preventing its normal function. Other types of NCC inhibitors may also exist, each with their own specific mechanism of action.
It's important to note that the use of NCC inhibitors should be done under the supervision of a healthcare professional, as they may have potential side effects and interactions with other medications. Additionally, individual responses to NCC inhibitors may vary, and dosage adjustments may be necessary based on a patient's specific condition and response to treatment.
Drug Target R&D Trends for methyclothiazide
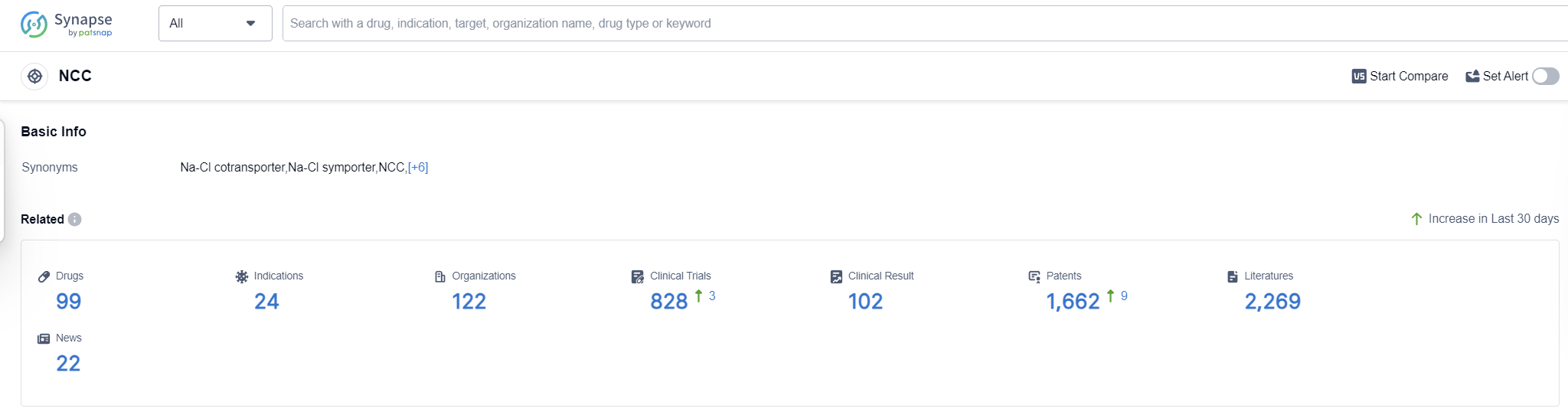
The NCC, or the Sodium-Chloride Cotransporter, plays a crucial role in the human body by facilitating the transport of sodium and chloride ions across cell membranes. This cotransporter is primarily found in the kidneys, where it helps regulate the reabsorption of sodium and chloride from the urine back into the bloodstream. By controlling the balance of these ions, the NCC contributes to the regulation of blood pressure and fluid balance in the body. Dysfunction of the NCC can lead to various health conditions, such as hypertension and electrolyte imbalances, highlighting its significance in maintaining overall physiological homeostasis.
According to Patsnap Synapse, as of 16 Sep 2023, there are a total of 99 NCC drugs worldwide, from 122 organizations, covering 24 indications, and conducting 828 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of target NCC shows that Novartis AG is the leading company with the highest number of approved drugs. The R&D progress of the companies under this target varies, with ongoing efforts in different stages of development. Hypertension is the most common indication for the approved drugs, and small molecule drugs are progressing rapidly. The United States and China are the countries with the highest number of approved drugs, indicating significant progress in these regions. Further analysis and additional information would be required to provide a more detailed assessment of the future development of target NCC.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Methyclothiazide is a small molecule drug developed by AbbVie, Inc. It is primarily used for the treatment of hypertension and targets the NCC in the body. With approvals in the global markets, Methyclothiazide has demonstrated its effectiveness and safety. Its first approval in the United States in 1960 signifies its long-standing presence in the field of biomedicine.