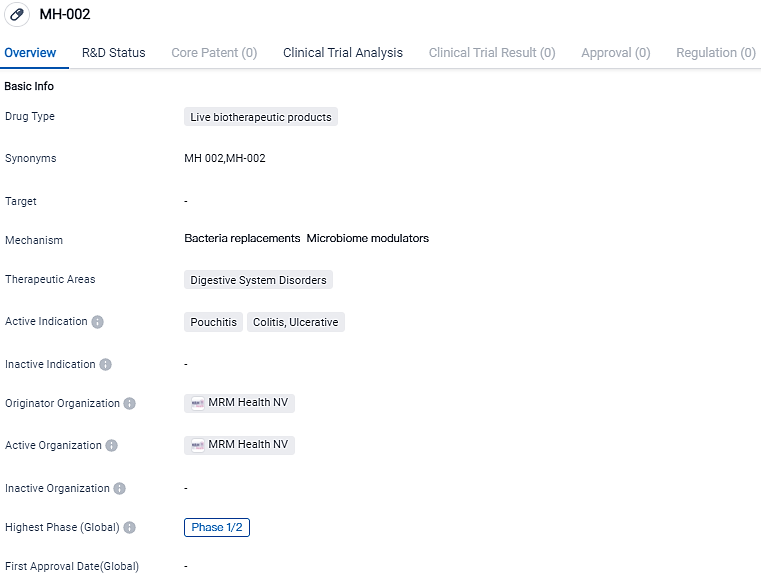
MRM Health showcases encouraging findings from its Phase 2a Clinical Trial of MH002 for Ulcerative Colitis
MRM Health NV, a biopharmaceutical firm aims at advancing next-gen live microbiome consortia therapies, has announced encouraging preliminary outcomes from its Phase 2a clinical study of MH002 against mild-to-moderate Ulcerative Colitis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The MH002-UC-201 experiment by MRM Health was conducted as a multi-center, double-blind, randomized, placebo-controlled test on 45 UC patients across numerous medical facilities in Belgium, Poland, and the Czech Republic. The experiment aimed to assess safety, preliminary effectiveness, and the operational impacts of MH002 over two periods of eight weeks each.
Séverine Vermeire, the principal investigator of the MH002-UC-201 experiment, and also a Professor of Medicine at KU Leuven, Belgium, stated, "These preliminary findings show that MH002 is safe and easily accepted by patients, and it may be effective in UC patients with mild to moderate severity who have not adequately responded to primary treatment. There is a significant absence of treatments for UC patients, particularly in this population, so I look forward to conducting the full analysis of the data and following MH002’s progression through the clinic.”
Adding to these comments, Bruce Sands, a Professor of Medicine at the Icahn School of Medicine at Mount Sinai, New York, and a compensated advisor to MRM Health, said: "MH002's mechanism of operation and its anti-inflammatory properties in UC seem quite promising and could offer a highly favourable benefit to risk ratio, subject to validation through larger-scale studies. The preliminary results also hint that this formulation could be applied to a broader spectrum of other inflammatory bowel diseases, such as pouchitis, where MH002 is currently under examination."
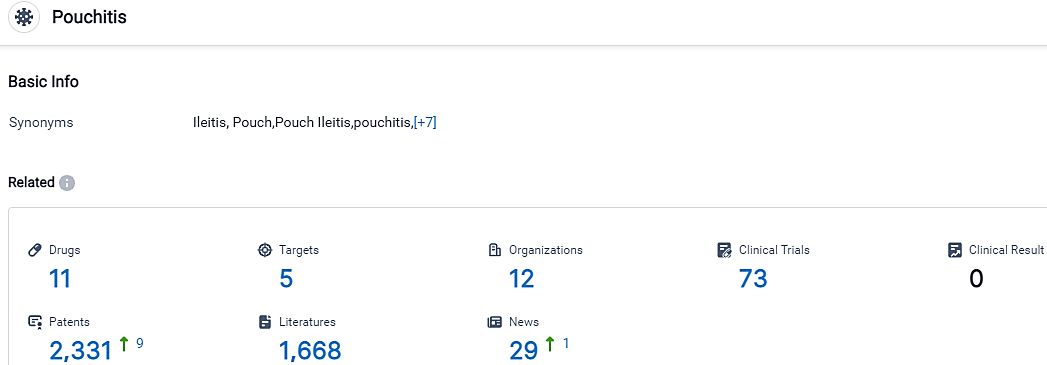
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
According to the data provided by the Synapse Database, As of September 22, 2023, there are 11 investigational drugs for the Pouchitis, including 5 targets,12 R&D institutions involved, with related clinical trials reaching 73,and as many as 2331 patents.
MH-002 is a live biotherapeutic product being developed by MRM Health NV for the treatment of digestive system disorders, specifically pouchitis, colitis, and ulcerative conditions. Full analysis and presentation of the data is expected by the end of 2023. The Company has initiated to progress the program into Phase 2/3 development.