Narsoplimab by Omeros Demonstrates Improved Survival in TA-TMA Patients, Meets Key Trial Milestone
Omeros Corporation (Nasdaq: OMER) has disclosed that a separate statistical team has finalized the primary statistical evaluation as per the agreement with the FDA regarding narsoplimab. This monoclonal antibody is Omeros' pioneering product designed to inhibit the lectin pathway of complement. It is intended for treating thrombotic microangiopathy (TA-TMA) related to hematopoietic stem cell transplants (HSCT), a severe and potentially fatal complication affecting both adults and children undergoing HSCT.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The study evaluated overall survival rates among 28 TA-TMA patients from OMS721-TMA-001, Omeros' earlier pivotal trial using narsoplimab for TA-TMA, and compared these rates to over 100 similarly high-risk TA-TMA patients from an external registry of HSCT patients who were not treated with narsoplimab. The primary endpoint was achieved, as patients in the OMS721-TMA-001 trial exhibited clinically significant and statistically relevant improvement in overall survival, reflected in a hazard ratio of 0.32 (95% confidence interval: 0.23 to 0.44) with a p-value below 0.00001, when compared to the registry cohort. Based on this data, Omeros intends to promptly resubmit its narsoplimab Biologics License Application (BLA) for TA-TMA to the FDA. Omeros aspires to have narsoplimab, an agent that targets the MASP-2 enzyme in the lectin pathway, recognized as the first approved therapy for TA-TMA.
Dr. Alessandro Rambaldi, a Professor of Hematology at the University of Milan and Head of the Hematology and Bone Marrow Transplant Unit at ASST Papa Giovanni XXIII in Bergamo, Italy, remarked, "The findings of this comparative analysis on the survival of TA-TMA patients treated with narsoplimab are remarkable. As one of the investigators in the pivotal trial and having utilized narsoplimab in numerous severely ill TA-TMA patients via the expanded access program, we have witnessed first-hand the significant advantages of narsoplimab for these high-risk individuals. Though we do not administer C5 inhibitors to our transplant patients, nearly 50 individuals in the expanded access program were treated with narsoplimab after either failing or discontinuing C5 inhibitors and other off-label therapies, with 46 percent of adults and 50 percent of children experiencing a resolution of TA-TMA, which is impressive. There is an urgent need for an approved treatment for TA-TMA patients, and we remain optimistic that narsoplimab will be made available soon."
At the end of last month, Omeros disclosed that it had received the FDA's feedback on the statistical analysis plan (SAP) for the primary comparative analysis of overall survival, starting from the initial dose among the 28 TA-TMA patients treated with narsoplimab in the pivotal OMS721-TMA-001 trial versus the adjusted overall survival of over 100 TA-TMA patients from the external control registry, who did not receive narsoplimab. The two groups shared similar demographics, diagnostic standards, baseline characteristics, underlying illnesses, conditioning regimens, and transplant methodologies. All patients in both groups were classified as high-risk for mortality, based on definitions provided by an international expert panel established to create consensus on diagnostic and prognostic criteria, which included representatives from the American Society for Transplantation and Cellular Therapy, the Center for International Bone Marrow Transplant Research, the Asia-Pacific Blood and Marrow Transplantation Group, and the European Society for Blood and Marrow Transplantation. Following the FDA’s guidelines, the independent statistical team integrated the recommendations into the final SAP, carried out and verified the primary analysis, and subsequently communicated the results to Omeros.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
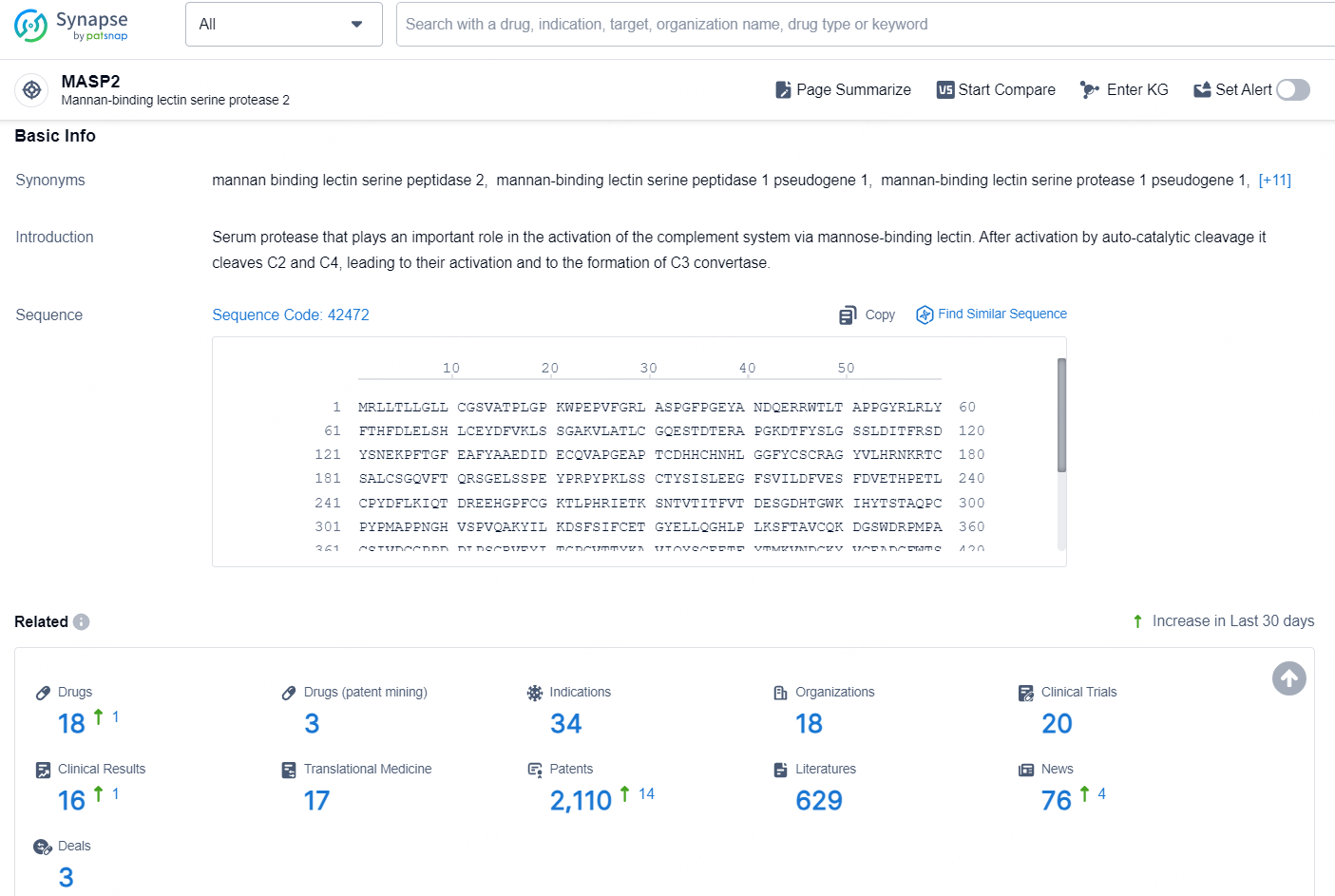
According to the data provided by the Synapse Database, As of December 24, 2024, there are 18 investigational drugs for the MASP2 target, including 34 indications, 18 R&D institutions involved, with related clinical trials reaching 20, and as many as 2110 patents.
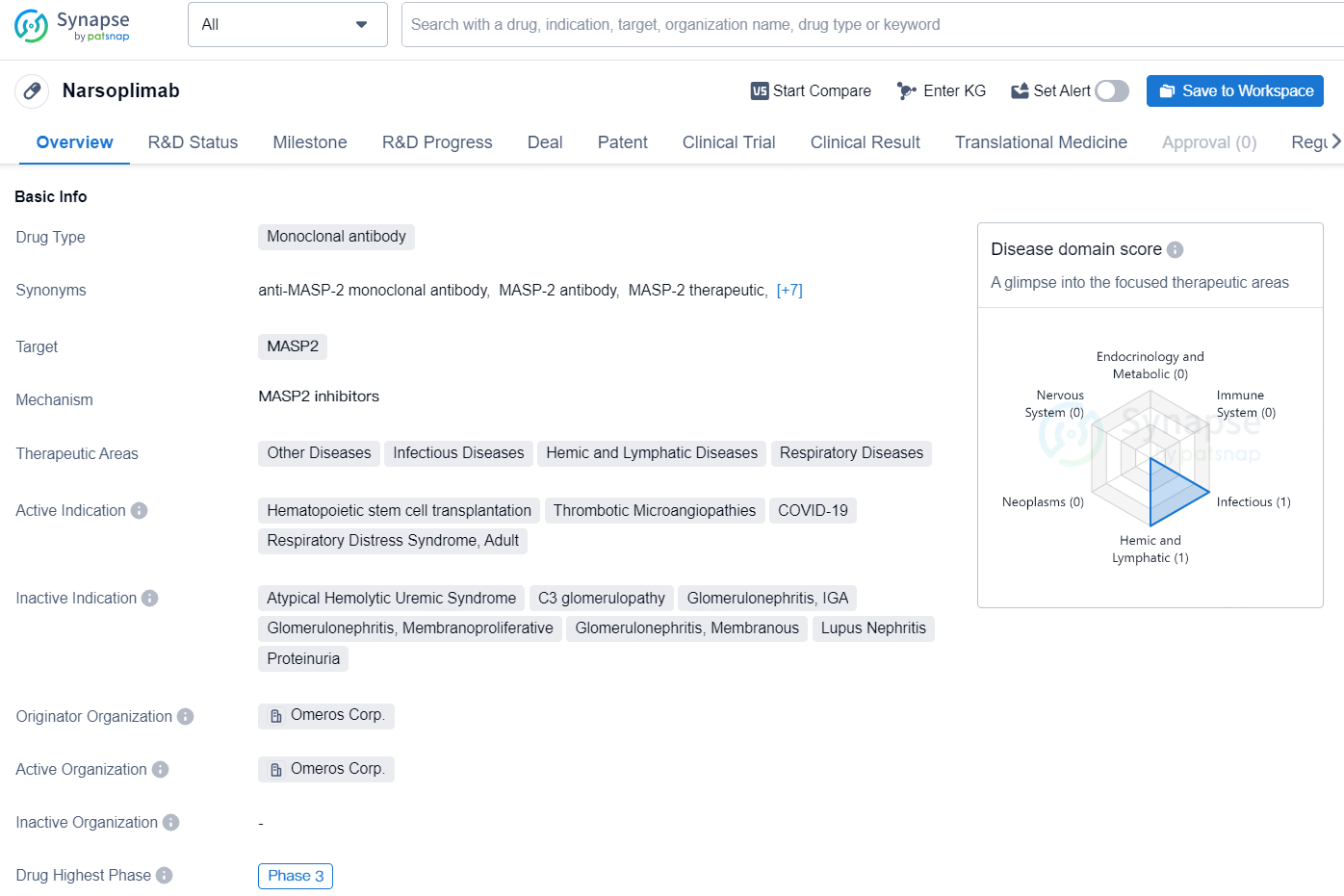
Narsoplimab is a monoclonal antibody drug developed by Omeros Corp., targeting the MASP2 protein. The drug is being developed for various therapeutic areas including Other Diseases, Infectious Diseases, Hemic and Lymphatic Diseases, and Respiratory Diseases. The active indications for Narsoplimab include Hematopoietic stem cell transplantation, Thrombotic Microangiopathies, COVID-19, and Respiratory Distress Syndrome in adults. The drug is currently in Phase 3, which is the highest phase of development globally.