Revolutionizing Cancer Treatment: The Emergence and Impact of KRAS G12C Inhibitors
KRAS G12C is a specific mutation of the KRAS gene that plays a crucial role in the development and progression of certain cancers. This mutation occurs in the KRAS protein, which is involved in regulating cell growth and division. In the human body, the KRAS G12C mutation leads to the overactivation of signaling pathways that promote uncontrolled cell proliferation and survival. This mutation is particularly prevalent in lung and colorectal cancers, making it an attractive target for therapeutic interventions. Efforts are underway to develop drugs that specifically inhibit the KRAS G12C mutation, with the aim of effectively treating these types of cancers.
Currently, there are two KRAS G12C inhibitors approved for marketing, sotorasib and Adagrasib.
On May 29, 2021, the U.S. FDA announced the accelerated approval of Lumakras (sotorasib, AMG510) developed by Amgen for the treatment of non-small cell lung cancer patients carrying the KRAS G12C mutation. The FDA’s approval is based on data from the AMG510 CodeBreaK 100 Phase 2 clinical trial (NCT03600883). This trial included 124 patients with advanced non-small cell lung cancer with the KRAS G12C mutation. The results showed an objective response rate (ORR) of 37.1%, including 3 complete responses and 43 partial responses, and a disease control rate of 80.6%. The median duration of response was 10 months. Notably, 81% of patients experienced tumor shrinkage (n = 101/124), and the average best tumor shrinkage among all patients responding to sotorasib treatment was 60%!
Adagrasib (MRTX849) is the second marketed, orally optimized inhibitor specific for the KRAS G12C mutation. It irreversibly and selectively binds to KRAS G12C in its inactive state, preventing it from sending cell growth signals and causing cancer cell death. In April 2023, updated data from the Phase 2 study KRYSTAL-1 (NCTO3785249) was presented at the ASCO Annual Meeting, showing strong activity of this drug against various solid tumors, including pancreatic cancer, biliary system tumors, appendiceal cancer, ovarian cancer, etc. The results showed a total objective response rate (ORR) of 35.1% and a disease control rate (DCR) of up to 86.0%, meaning that nearly 90% of patients had their tumors shrink or stabilize to varying degrees.
At the 2023 AACR conference, new data from the Phase Ib study GDC-6036 was released. 29 patients with advanced or metastatic KRAS G12C positive colorectal cancer were enrolled. These patients were all late-stage patients who had failed at least two treatment regimens and received divarasib and cetuximab treatment. The results showed that Divarasib has good clinical activity and controllable safety. 66% (n=19/29) of patients achieved unconfirmed overall responses, and 62% (n=18/29) of patients achieved confirmed overall responses. This means that over 60% of late-stage patients had significant tumor shrinkage of more than 30%! More notably, among the 5 patients who had previously received KRAS G12C inhibitor treatment, 3 patients responded to divarasib treatment, meaning that patients resistant to treatment now have a new “life-extending” option.
Overall, the target KRAS G12C presents a competitive landscape with multiple companies, various indications, and different drug types. The future development of drugs targeting KRAS G12C holds great potential, especially in countries like China, where significant progress has been made.
How do they work?
KRAS G12D inhibitors are a type of medication or therapeutic agents that specifically target and inhibit the activity of the KRAS G12D mutation. KRAS is a gene that plays a crucial role in regulating cell growth and division. However, mutations in the KRAS gene, such as the G12D mutation, can lead to uncontrolled cell growth and contribute to the development and progression of certain cancers.
From a biomedical perspective, KRAS G12D inhibitors are designed to specifically target the KRAS G12D mutation and block its signaling pathway. By inhibiting the activity of this mutated gene, these inhibitors aim to disrupt the abnormal cell growth and division, ultimately suppressing the growth of cancer cells. This targeted approach holds promise for the treatment of cancers that harbor the KRAS G12D mutation, such as certain types of lung, colorectal, and pancreatic cancers.
It is important to note that KRAS G12D inhibitors are still under development and clinical trials. While they show potential as a targeted therapy for specific cancers, further research is needed to evaluate their safety, efficacy, and potential side effects.
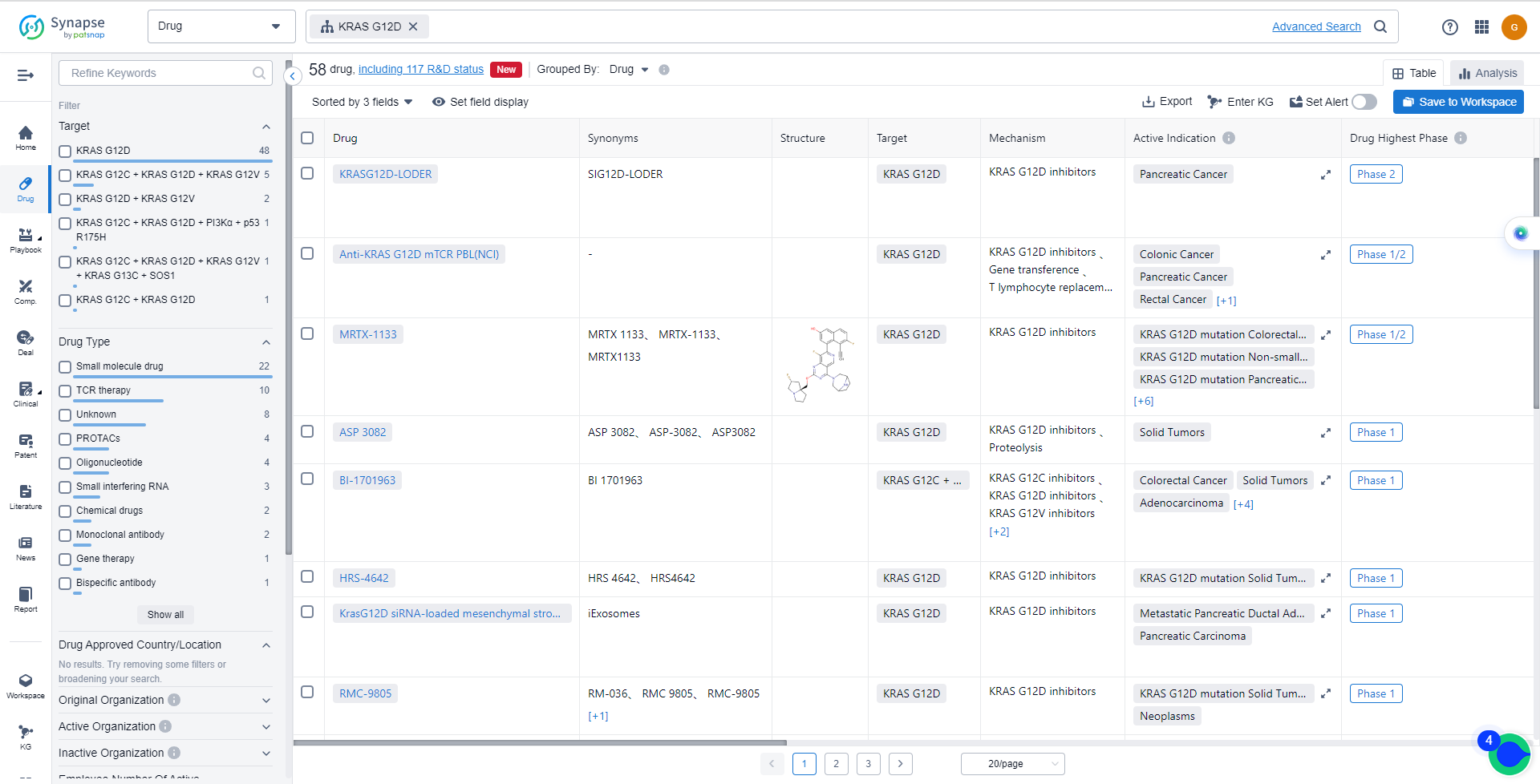
List of KRAS G12C Inhibitors
The currently marketed KRAS G12C inhibitors include:
- KRASG12D-LODER
- Anti-KRAS G12D mTCR PBL(NCI)
- MRTX-1133
- ASP 3082
- BI-1701963
- HRS-4642
- RMC-9805
- UA022
- DCTY-1102
- DN-022150
For more information, please click on the image below.
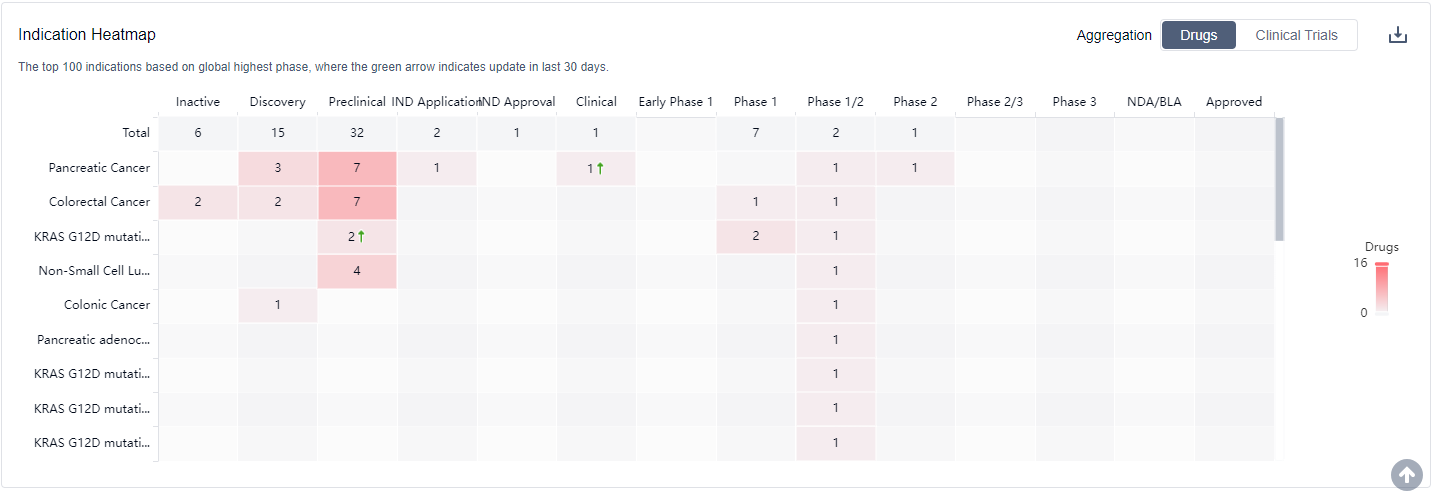
What are KRAS G12C inhibitors used for?
KRAS G12C inhibitors are mainly used in a variety of solid tumors such as non-small cell lung cancer, pancreatic cancer, biliary system tumors, appendix cancer, ovarian cancer, etc. please click on the image below to log in and search.
How to obtain the latest development progress of KRAS G12C inhibitors?
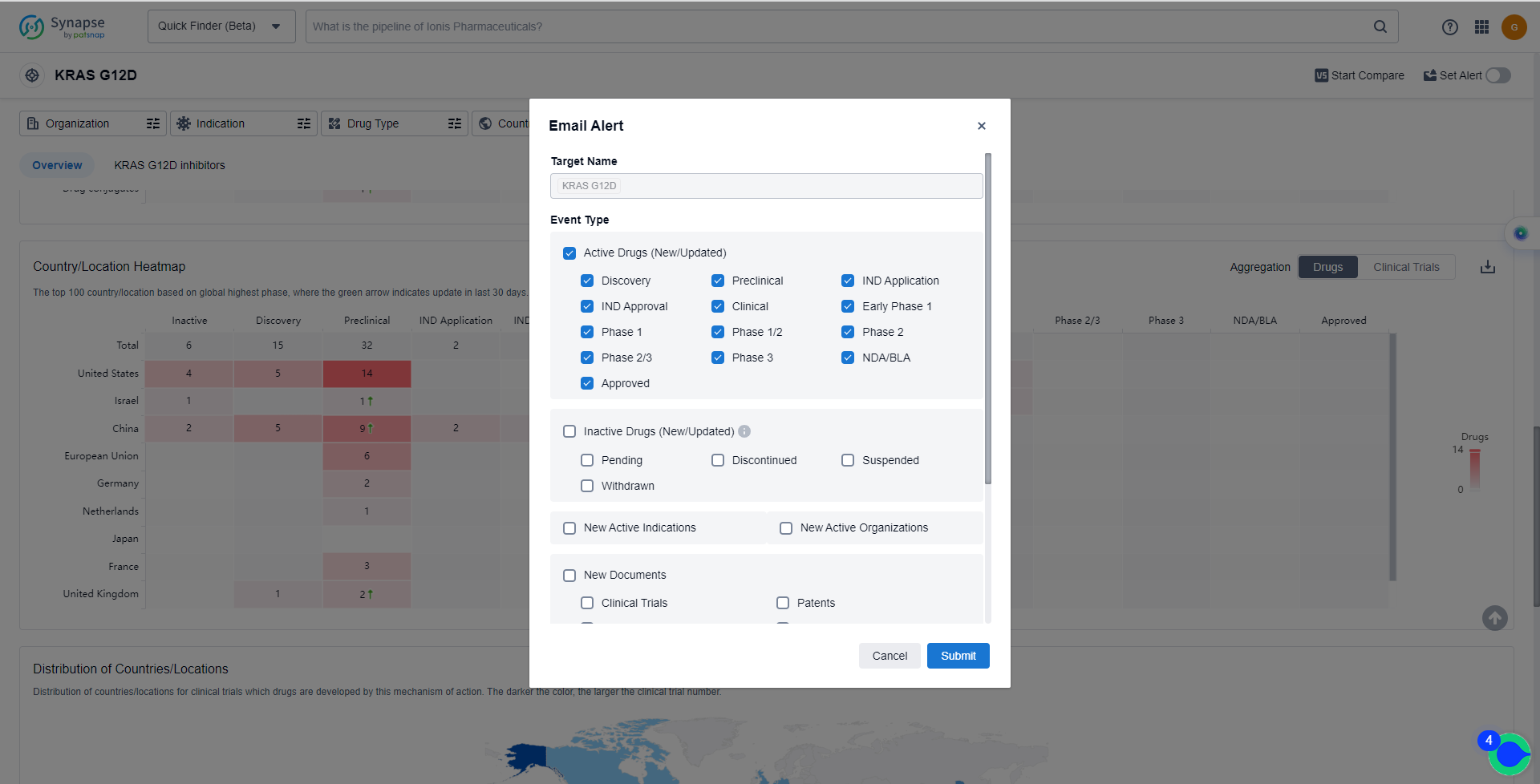
In the Synapse database, you can keep abreast of the latest research and development advances of KRAS G12C inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!