Pharmaceutical Insights: toremifene citrate's R&D Progress and its Mechanism of Action on Drug Target
Toremifene citrate's R&D Progress
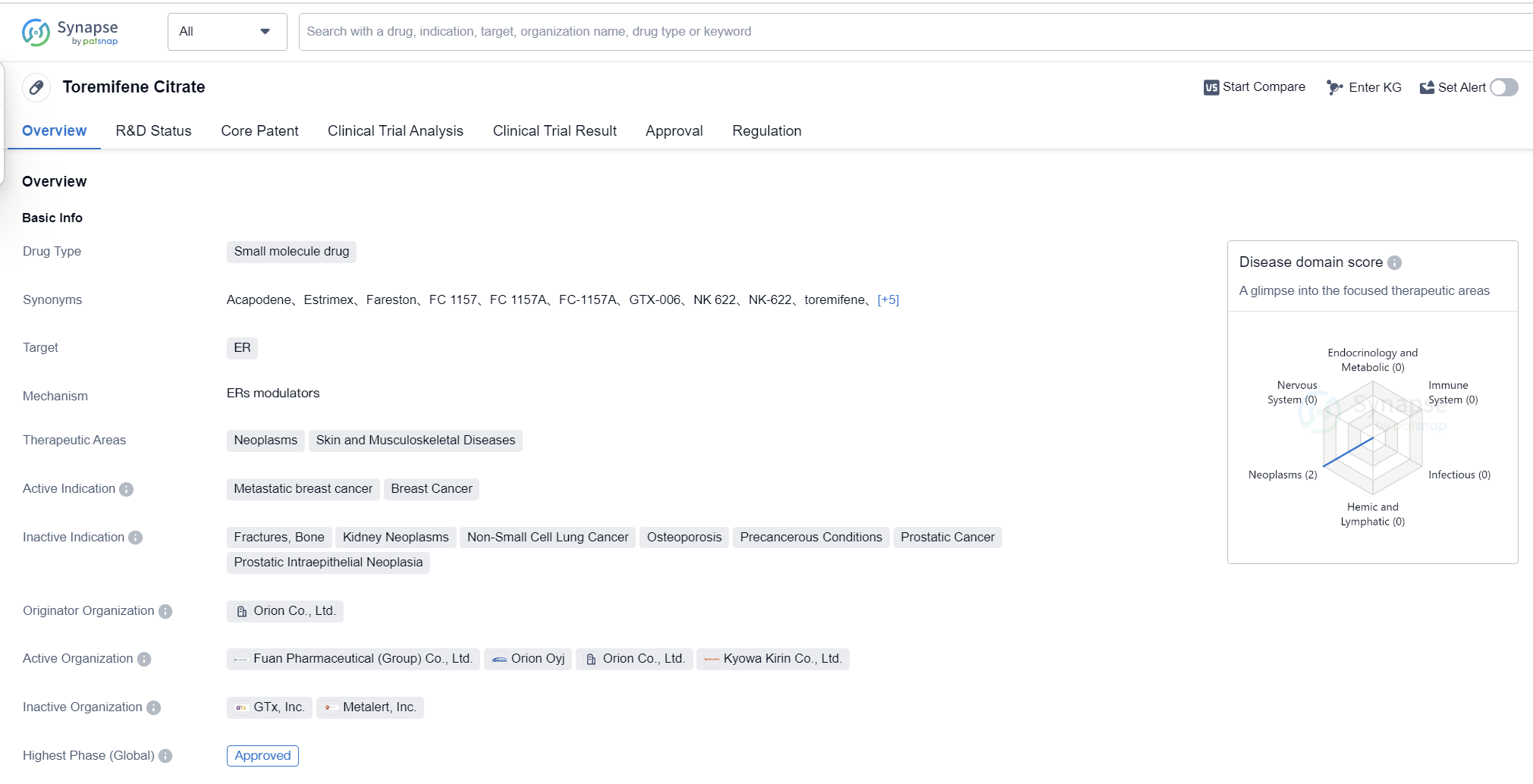
Toremifene Citrate is a small molecule drug that falls under the category of biomedicine. It primarily targets the estrogen receptor (ER) and is used in the treatment of neoplasms, skin diseases, and musculoskeletal diseases. The drug has been approved for the treatment of metastatic breast cancer and breast cancer.
The originator organization of Toremifene Citrate is Orion Co., Ltd., a pharmaceutical company based in Japan. The drug has reached the highest phase of development which is approved globally. It received its first approval in March 1995 in Japan, making it available for use in that country.
Toremifene Citrate is classified as an orphan drug, which means it is designed to treat rare diseases or conditions that affect only a small number of people. Such drugs typically receive special regulatory incentives and exclusivity periods to encourage their development and availability.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for toremifene citrate: ERs modulators
ERs modulators are compounds or substances that can interact with and modulate the activity of estrogen receptors (ERs). Estrogen receptors are proteins found in cells that are activated by the hormone estrogen. They play a crucial role in various physiological processes, including the development and maintenance of female reproductive organs, regulation of the menstrual cycle, and the growth and differentiation of breast tissue.
ERs modulators can have different effects on estrogen receptors depending on their specific properties. Some modulators can act as agonists, meaning they bind to the receptors and activate them, mimicking the effects of estrogen. These agonists can be used in hormone replacement therapy to alleviate symptoms of menopause or to treat certain conditions such as osteoporosis.
On the other hand, some ERs modulators act as antagonists, blocking the binding of estrogen to the receptors and preventing their activation. These antagonists are used in the treatment of hormone-dependent cancers, such as breast cancer, where blocking the estrogen receptor signaling can inhibit the growth of cancer cells.
Additionally, there are selective ERs modulators (SERMs) that can exhibit both agonistic and antagonistic effects depending on the target tissue. For example, a SERM may act as an estrogen agonist in bone tissue, promoting bone density, while acting as an estrogen antagonist in breast tissue, preventing the growth of cancer cells.
Overall, ERs modulators are important tools in biomedicine for regulating estrogen receptor activity and can have therapeutic applications in various conditions related to hormone regulation and hormone-dependent diseases.
Drug Target R&D Trends for toremifene citrate
The endoplasmic reticulum (ER) is a vital organelle found in eukaryotic cells, including human cells. It plays a crucial role in protein synthesis, folding, and transport within the cell. The ER consists of two distinct regions: the rough ER, which is studded with ribosomes and involved in the production of proteins, and the smooth ER, which is responsible for lipid metabolism and detoxification processes. Additionally, the ER is involved in calcium storage and release, which is essential for various cellular functions. Overall, the ER is a multifunctional organelle that contributes significantly to the proper functioning of cells and the overall health of the human body.
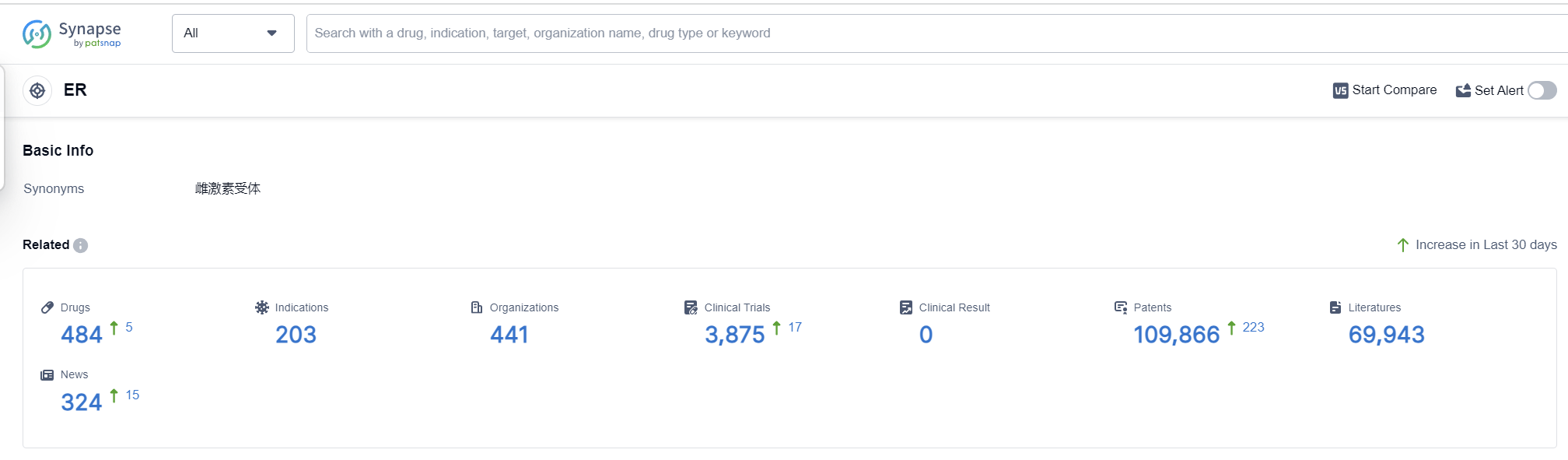
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 484 ER drugs worldwide, from 441 organizations, covering 203 indications, and conducting 3875 clinical trials.
The current competitive landscape for the target ER is characterized by the dominance of companies like Bayer AG, Pfizer Inc., and Mochida Pharmaceutical Co., Ltd. These companies have made significant progress in terms of drug development, with Bayer AG having the highest number of approved drugs. Indications such as contraception, osteoporosis (postmenopausal), and breast cancer have seen substantial advancements in drug development. Small molecule drugs and PROTACs are the drug types that are progressing most rapidly, indicating intense competition and innovation. The United States, China, and the European Union are the countries/locations that are developing fastest, with the United States leading in terms of approved drugs. China has shown notable progress, particularly in the approved and preclinical stages. Overall, the target ER presents a competitive landscape with promising future development potential.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Toremifene Citrate is a small molecule drug that targets the estrogen receptor and is used in the treatment of neoplasms, skin diseases, and musculoskeletal diseases. It has been approved for the treatment of metastatic breast cancer and breast cancer. The drug was first approved in Japan in 1995 and is regulated as an orphan drug. Its originator organization is Orion Co., Ltd.