Preliminary Success for Janux Therapeutics’ Prostate and Solid Cancer Drugs JANX007 and JANX008
Janux Therapeutics, Inc., an enterprise focused on the clinical development of innovative immunotherapy treatments, utilizing its unique platforms – the Tumor Activated T Cell Engager and Tumor Activated Immunomodulator – has reported encouraging progress in the latest clinical findings across all its treatment investigations.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 Recent clinical findings offer promising evidence of the safety and effectiveness for JANX007 in treating metastatic castration-resistant prostate malignancies, and JANX008 demonstrates similar promise in advanced-stage solid malignancies. These results validate the potential of the TRACTr technology in scenarios where numerous other methods have been unsuccessful due to safety concerns or inefficacy, according to Janux Therapeutics President and CEO, Dr. David Campbell.
Recent clinical findings offer promising evidence of the safety and effectiveness for JANX007 in treating metastatic castration-resistant prostate malignancies, and JANX008 demonstrates similar promise in advanced-stage solid malignancies. These results validate the potential of the TRACTr technology in scenarios where numerous other methods have been unsuccessful due to safety concerns or inefficacy, according to Janux Therapeutics President and CEO, Dr. David Campbell.
Dr. Campbell further highlighted the versatility of the TRACTr technology for addressing various difficult solid tumor types where traditional T cell engager strategies have not been effective. He expressed enthusiasm about continuing the progression of these clinical studies and delving into more T cell engager targets, aiming to meet the pressing health needs of cancer sufferers, particularly those in advanced stages.
JANX007 is undergoing a Phase 1a trial targeting men with severely advanced prostate cancer. Individuals in this study have undergone extensive prior treatments, averaging more than four different therapy types. By February 12, 2024, a total of 23 trial participants had received JANX007 during the dose escalation stage of the Phase 1a trial.
JANX008 is also involved in a Phase 1a study, focusing on individuals with advanced solid tumors that exhibit a high expression of EGFR, including specific types such as colorectal, head and neck squamous cell, non-small cell lung, and renal cell cancers.
As of February 12, 2024, 11 individuals with advanced disease stages, each having received multiple treatments, have been admitted into the study. Notable clinical activity has been seen, along with a continuation of a favorable safety track record. Remarkably, an NSCLC patient receiving JANX008 weekly at a dose of 0.15mg showed a confirmed partial response by RECIST standards with complete regression of the primary pulmonary lesion and resolution of hepatic metastases, while avoiding cytokine release syndrome (CRS) or treatment-related adverse events (TRAEs). This patient's partial response is still apparent in their 18-week checkup. Another patient, suffering from RCC, has seen a 12% decrease in tumor size alongside substantial clinical advantages, encountering a mild CRS, Grade 1 in severity.
The initial safety data for JANX008 aligns with TRACTr's intent for targeted tumor activation to prevent toxicity in healthy cells, despite the target's wider expression. In those enrolled, doses upped to 1.25mg have been administered, which surpasses the presumed highest dose that the baseline T cell engager can tolerate. Of these, only two participants experienced a Grade 1 CRS, with no observations of CRS of Grade 2 severity or more.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
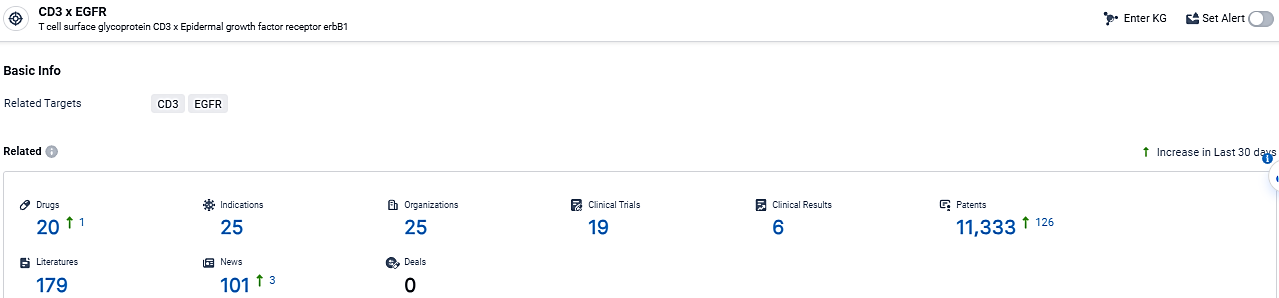
According to the data provided by the Synapse Database, As of February 29, 2024, there are 20 investigational drugs for the CD3 x EGFR target, including 25 indications,25 R&D institutions involved, with related clinical trials reaching 19, and as many as 11333 patents.
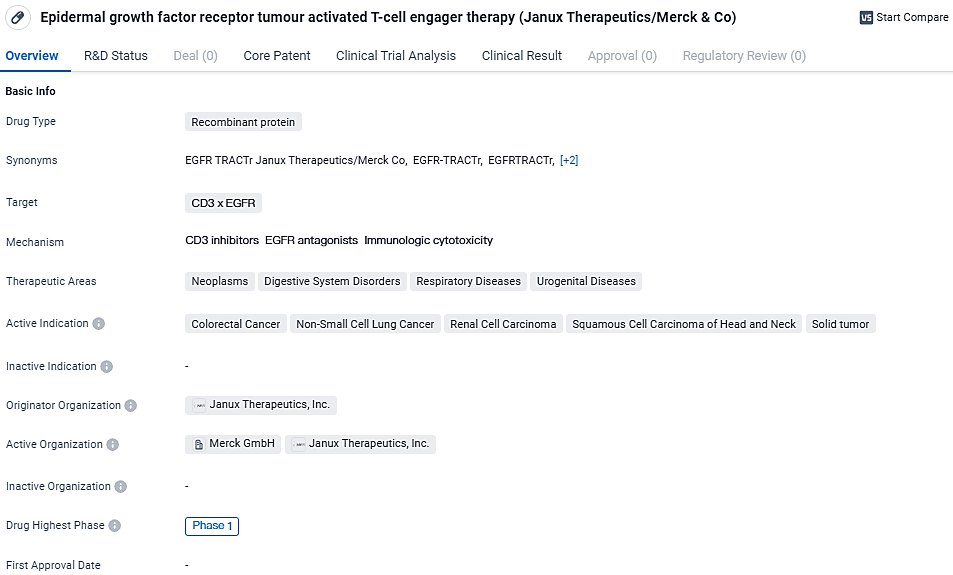
JANX007 is a recombinant protein that targets the CD3 x EGFR receptors. This therapy is primarily used in the treatment of various neoplasms, digestive system disorders, respiratory diseases, and urogenital diseases. The active indications for this therapy include colorectal cancer, non-small cell lung cancer, renal cell carcinoma, squamous cell carcinoma of the head and neck, and solid tumors. These are all serious and potentially life-threatening conditions, making the development of effective treatments crucial.