Progress in the Research and Development of Cannabinoid Receptor New Drugs
Throughout history, cannabis has been utilized as a medicinal substance, derived from plants of the Cannabis genus. Its principal constituents are Tetrahydrocannabinol (THC) and Cannabidiol (CBD). Cannabinoids are a group of terpenophenolic compounds discovered within cannabis and also occur naturally in the nervous and immune systems of animals. In the 1980s, researchers identified two G protein-coupled receptors that exhibit specific responses to cannabinoids: the cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2).
Cannabinoid receptors and endogenous cannabinoids are not only involved in regulating numerous physiological functions of the central and peripheral nervous systems but also play a role in regulating the physiological functions of many peripheral sites in the human body, including the endocrine system, immune system, gastrointestinal tract, cardiovascular system, and reproductive system.

CB1 receptors, although primarily found in the brain, are also expressed in the spleen, lungs, thymus, heart, and blood vessels. Within the central nervous system, their expression is highest at the axons and presynaptic terminals of neurons in the amygdala, hippocampus, cortex, basal ganglia, and cerebellum. They are closely associated with GABAergic (inhibitory) and glutamatergic (excitatory) neurons, mediating the sustained release of inhibitory and excitatory neurotransmitters.
CB2 receptors are most abundantly found in tissues with immune functions outside the brain (such as the spleen, tonsils, bone marrow, pancreas, peripheral blood leukocytes) and within the brain (astroglia and microglia). They play a role in regulating immune cell migration and cytokine release, and are also involved in the cardiovascular and respiratory systems, bones, gastrointestinal tract, liver, and reproductive system.
Cannabinoids can be categorized into three types based on their origin: endocannabinoids, phytocannabinoids, and synthetic cannabinoids.
Endocannabinoids are chemicals produced within the human body that attach to the same receptors (CB1 and CB2) as certain plant-derived cannabinoids. These endogenous chemical substances help in maintaining equilibrium and harmony in a healthy body. The two most extensively studied endocannabinoids, anandamide and 2-AG, along with the enzymes responsible for their metabolism, constitute the endocannabinoid system. The molecular targets of anandamide and 2-AG, along with other phytocannabinoids, are not just CB1 and CB2, but also include TRPR and GPR55. A key characteristic of endocannabinoids is that their precursors are present in lipid membranes, from which they can be synthesized through one or two rapid enzymatic steps and released into the extracellular space.
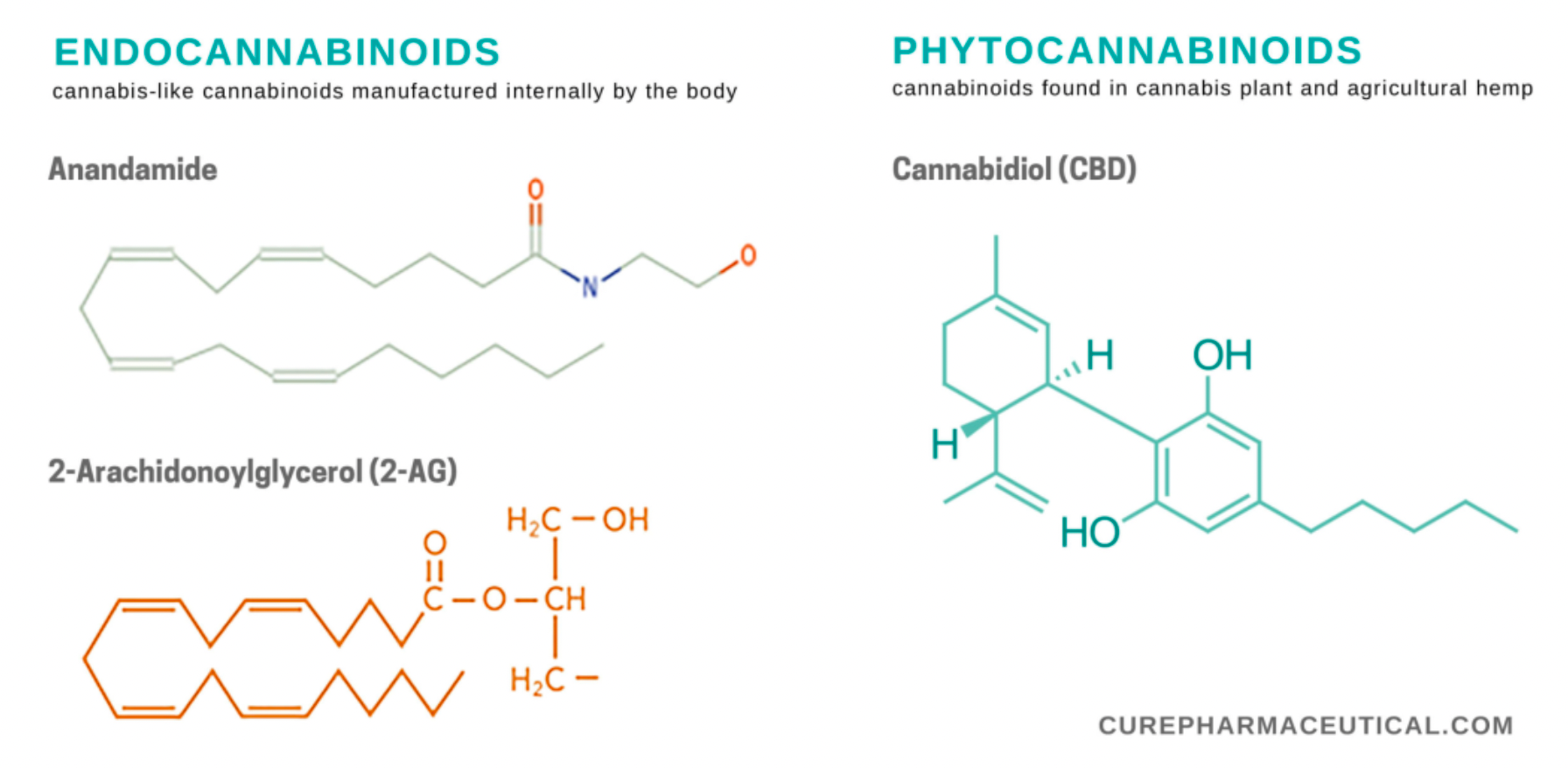
Phytocannabinoids are chemical substances found and extracted from the cannabis plant. The cannabis plant contains over 100 known phytocannabinoids, with the highest concentration typically found in the unfertilized flower buds of female plants. THC (Tetrahydrocannabinol) and CBD (Cannabidiol) are the most extensively studied phytocannabinoids. Currently, only one phytocannabinoid product has been approved by the U.S. FDA, which is a CBD oral solution approved for the treatment of seizures associated with rare forms of epilepsy. The term "medical marijuana," which is not FDA-approved, lacks a scientific definition but is generally understood to be a product containing phytocannabinoids from the cannabis plant, intended to alleviate the symptoms of diseases.
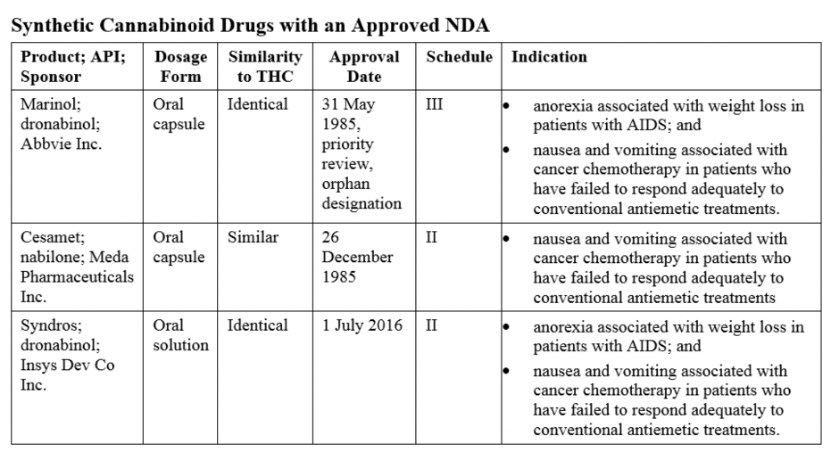
Synthetic cannabinoids are chemical substances created in a laboratory to mimic plant cannabinoids. The United States FDA has approved three synthetic cannabinoid products that imitate the plant cannabinoid THC. Synthetic cannabinoids may be more potent than tetrahydrocannabinol (THC) extracted from cannabis. The FDA-approved synthetic THC formulations Nabilone (sold under the brand name Cesamet) and Dronabinol (sold under the brand names Marinol and Syndros) are used for the treatment of nausea and vomiting associated with cancer chemotherapy; Dronabinol is also indicated for the treatment of anorexia associated with weight loss in patients with AIDS.
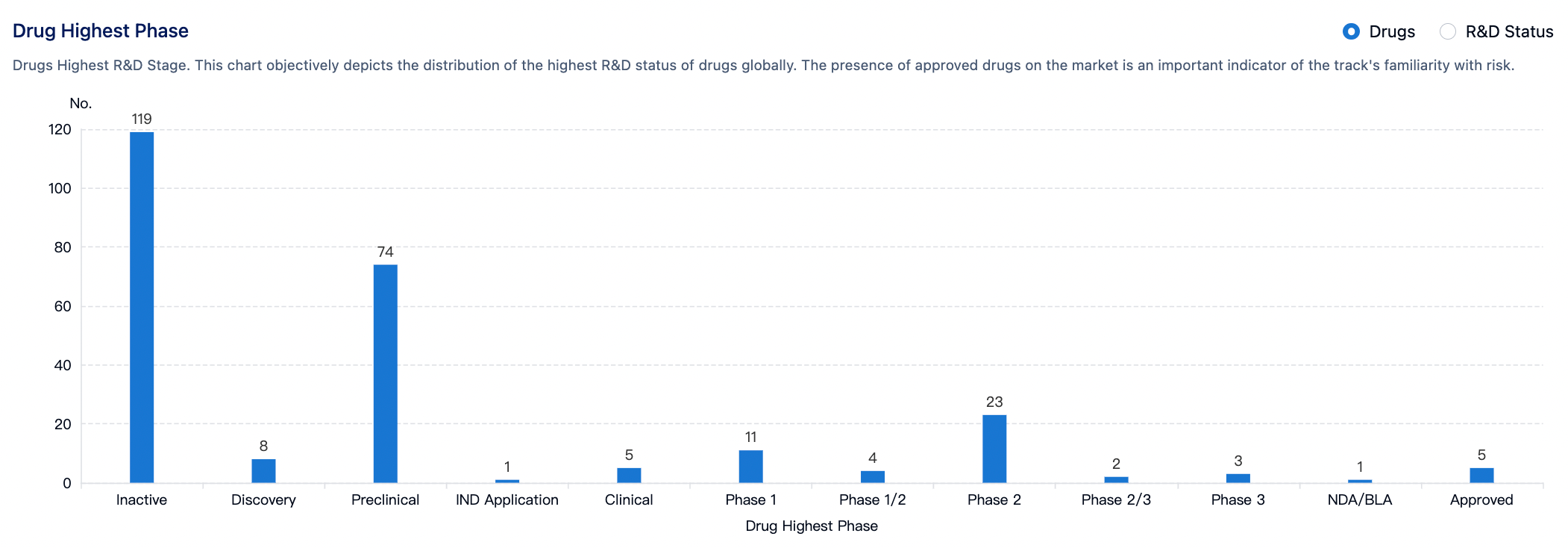
According to statistics from the Synapse database, there are a total of 256 drug development pipelines worldwide targeting the cannabinoid receptors (CB1 and CB2). Approximately 119 projects (46%) are at the non-research phase, while 74 pipelines (29%) are at the pre-clinical research stage. Five drugs have been approved for the market, including Cannabidiol, Dronabinol, and Rimonabant. Six drug candidates are in the clinical phase 2/3 or phase 3 study stages, and 23 are in clinical phase 2.
Apart from the development of subtype-selective therapeutic drugs for CB1 or CB2, some pharmaceutical research efforts focus on developing non-selective CB1/CB2 drug molecules and multi-target drugs that act on both CB1/2 and other receptors such as 5-HT1A and NMDA receptors.
Clinical indications and drug development opportunities targeting CB1 and CB2 receptors include: treatments for neurological disorders (such as neuropathic pain, epilepsy, Parkinson's disease, etc.), metabolic diseases (such as obesity, type 2 diabetes, non-alcoholic fatty liver disease, etc.), pain management (pain and neuropathy), immune system modulation (such as rheumatoid arthritis, inflammatory bowel disease, etc.), anti-cancer therapies, and interventions for substance addiction and mental health, among others. Novel therapeutics targeting CB1 and CB2 receptors may comprise agonists, antagonists, partial agonists, biased agonists, allosteric modulators, etc., to more precisely regulate the activity of the endocannabinoid system and reduce side effects.
Cannabidiol/Dronabinol (CBD/THC) is a medication developed by GW Pharmaceuticals for the treatment of sleep initiation and sleep disorders, which was first approved for sale in Germany in 2022.
Cannabidiol is an active cannabinoid found in cannabis, which can be extracted from cannabis or hemp plants. It is used to treat seizures associated with Lennox-Gastaut syndrome or Dravet syndrome, as well as relief for moderate to severe neuropathic pain or other pain symptoms. While its precise medical impact is under investigation, Cannabidiol has shown promise as an analgesic, anticonvulsant, muscle relaxant, anxiolytic, antipsychotic, and it has also shown neuroprotective, anti-inflammatory, and antioxidant activities.
Dronabinol is a synthetic Δ9-tetrahydrocannabinol (Δ⁹-THC) that functions through mild partial agonist activity on CB1 and CB2 receptors, producing effects known from cannabis consumption such as increased appetite, pain relief, and altered mood and cognitive processes. It has been approved in various countries and regions for treating anorexia associated with weight loss in AIDS patients, as well as for nausea and vomiting caused by chemotherapy in cancer patients who have not responded adequately to conventional antiemetic treatments.
GW Pharmaceuticals has established a world-leading cannabinoid science platform for the development of a deep, innovative portfolio of early and late-stage cannabinoid product candidates, along with highly specialized cultivation and manufacturing technologies. GW was also the first to develop and commercialize the world's first prescription medicine derived from the cannabis plant. Unlike CBD products or cannabis derivatives not approved by the FDA, GW's portfolio of regulator-approved products ensures that each of them undergoes a thorough evaluation of safety and efficacy through clinical trials, independent peer reviews, regulator approvals, and is consistently produced to the highest quality standards. In 2021, JAZZ Pharmaceuticals acquired GW Pharmaceuticals for $7.2 billion.
Rimonabant is an anorectic anti-obesity drug originally researched, developed, produced, and marketed by the company Sanofi-Aventis. It acts as a reverse agonist for the cannabinoid receptor CB1, with its primary mechanism of action being the reduction of appetite. Rimonabant was the first selective CB1 receptor blocker to be approved for use globally, and after its initial market approval in 2006, it received authorization in 38 countries including the European Union, Mexico, and Brazil. However, its application for market approval was rejected by the U.S. FDA due to potential risks of increased suicidal thoughts and depression.
Early clinical observations noted that cannabis stimulates appetite, suggesting the involvement of the endogenous cannabinoid system in the control of energy balance. Rimonabant was the first CB1 receptor antagonist to be marketed and was developed as an anti-obesity drug intended to decrease food intake by blocking central cannabinoid activity.
Around 2005-2006, Sanofi-Aventis and clinical research teams published articles in "The Lancet" (two papers) and "The New England Journal of Medicine," discussing the effects of Rimonabant on weight reduction and cardiovascular risk factors in overweight patients, the influence on metabolic risk factors in patients with overweight dyslipidemia, and the efficacy and tolerability in overweight or obese patients with type 2 diabetes.
The results indicate that for overweight or obese patients with Type 2 diabetes who exhibit inadequate glycemic control with metformin or sulfonylureas, a combined treatment of 20 mg/day Rimonabant with diet and exercise can lead to weight reduction and improvement in HbA1c, as well as a range of cardiovascular and metabolic risk factors, which is clinically significant.
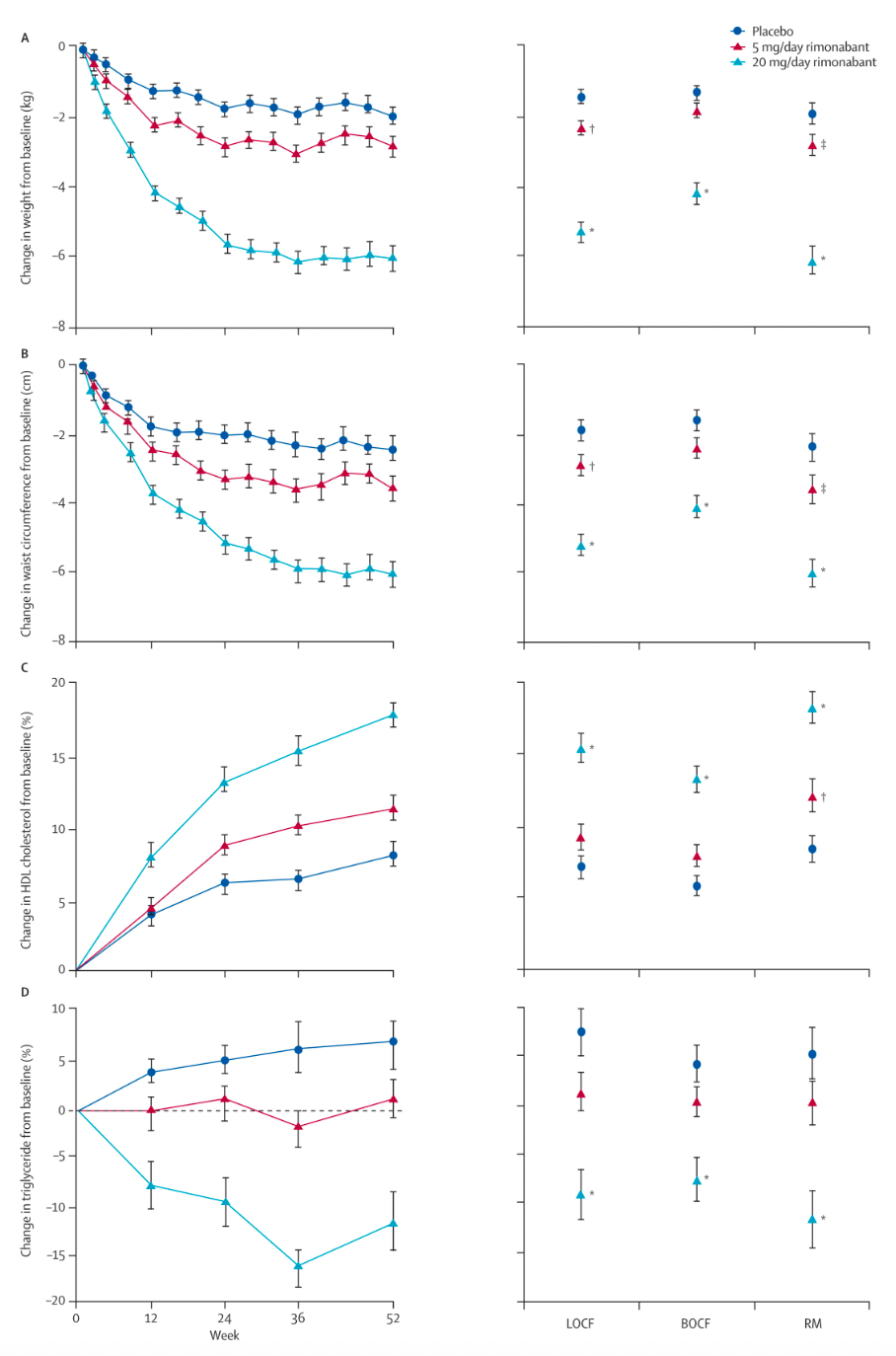
However, a meta-analysis of all published randomized controlled trials has shown that taking 20 mg of Rimonabant daily increases the risk of psychiatric adverse events (mood disorders including depressive disorders and anxiety). Adverse events associated with Rimonabant were significantly more frequent than with placebo, with severe adverse events occurring 1.4 times more often than with placebo. Patients taking Rimonabant were 2.5 times more likely to discontinue treatment due to depressive mood disorders compared to those receiving a placebo.
The U.S. FDA analyzed four major trials as well as unpublished studies and found that taking Rimonabant (20 mg/day) resulted in a higher incidence of psychiatric adverse reactions compared to placebo. Furthermore, it was reported that two patients taking Rimonabant died from suicide. The United States never approved the drug for the treatment of obesity. Subsequently, European regulatory authorities revoked the marketing authorization for Rimonabant.
Taranabant is a small molecule CB receptor inverse agonist originally developed by Merck & Co. It is reported that phase 3 clinical trial data for taranabant in overweight patients showed a weight reduction effect similar to that of Rimonabant. However, due to potential side effects and other reasons, Merck & Co. terminated the clinical development of taranabant.
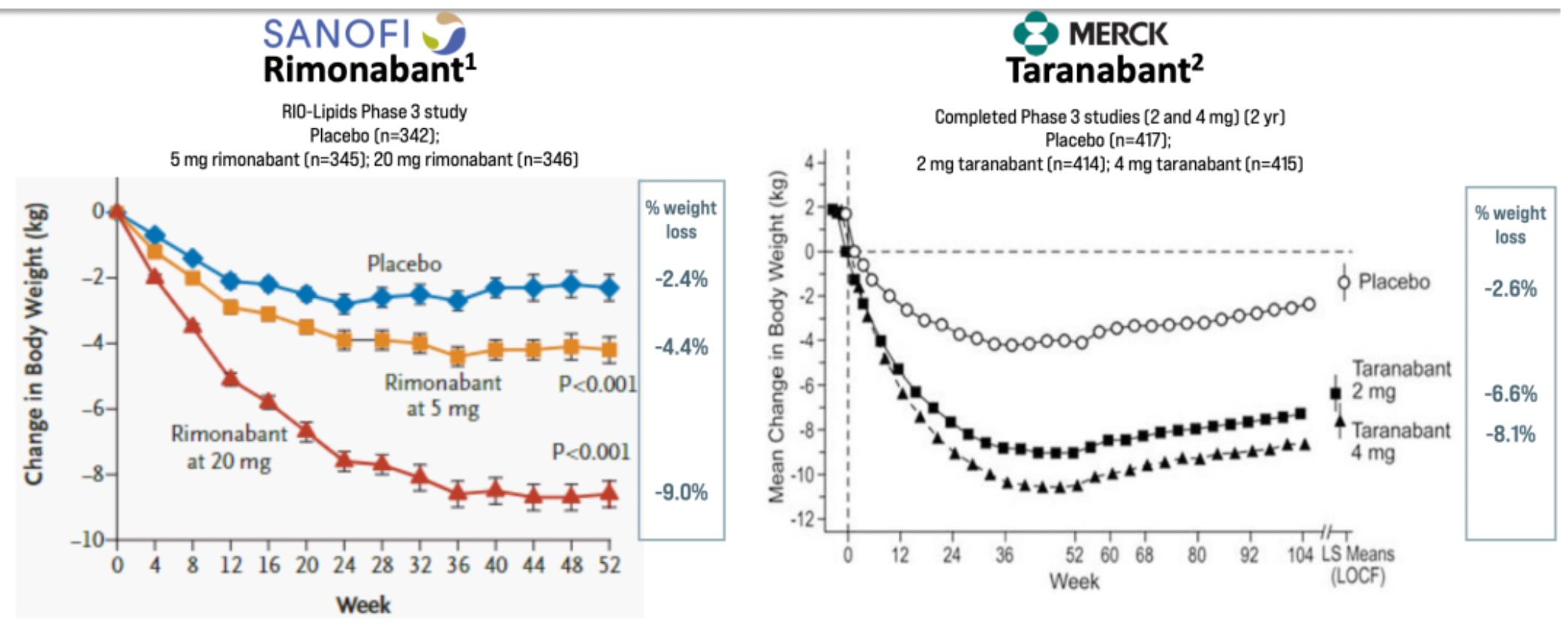
Subsequently, numerous clinical research projects associated with CB1 antagonists were discontinued as a result of this impact, but some products continue to be developed.
INV-202 is a CB1 receptor antagonist developed by Inversago Pharma. Learning from prior endeavors, Inversago Pharma's second-generation CB1 receptor antagonists are designed to reduce the permeability of the molecules through the blood-brain barrier, thereby confining the drug molecules to the peripheral system outside of the central nervous system.

According to a phase 1b clinical study report updated in 2023, INV-202 showed good tolerability with no serious or severe treatment-emergent adverse events noted; the most common side effects were related to the known gastrointestinal impacts of CB1 receptor blockade. INV-202 significantly reduced average body weight by 3.5 kilograms and also notably decreased waist circumference and body mass index. In August this year, Novo Nordisk and Inversago Pharma announced that Novo Nordisk has agreed to acquire Inversago for up to $1.075 billion in cash, subject to achieving certain development and commercial milestones.
Corbus Pharmaceuticals, another company dedicated to the development of drugs targeting the CB receptor system, has announced that it is working on developing a second-generation CB1 receptor antagonist, CRB-913, which is currently in the clinical declaration stage. Clinical pre-study data updated in November by the company indicate that CRB-913 has a low entry rate into the brain yet demonstrates significant weight loss effects.
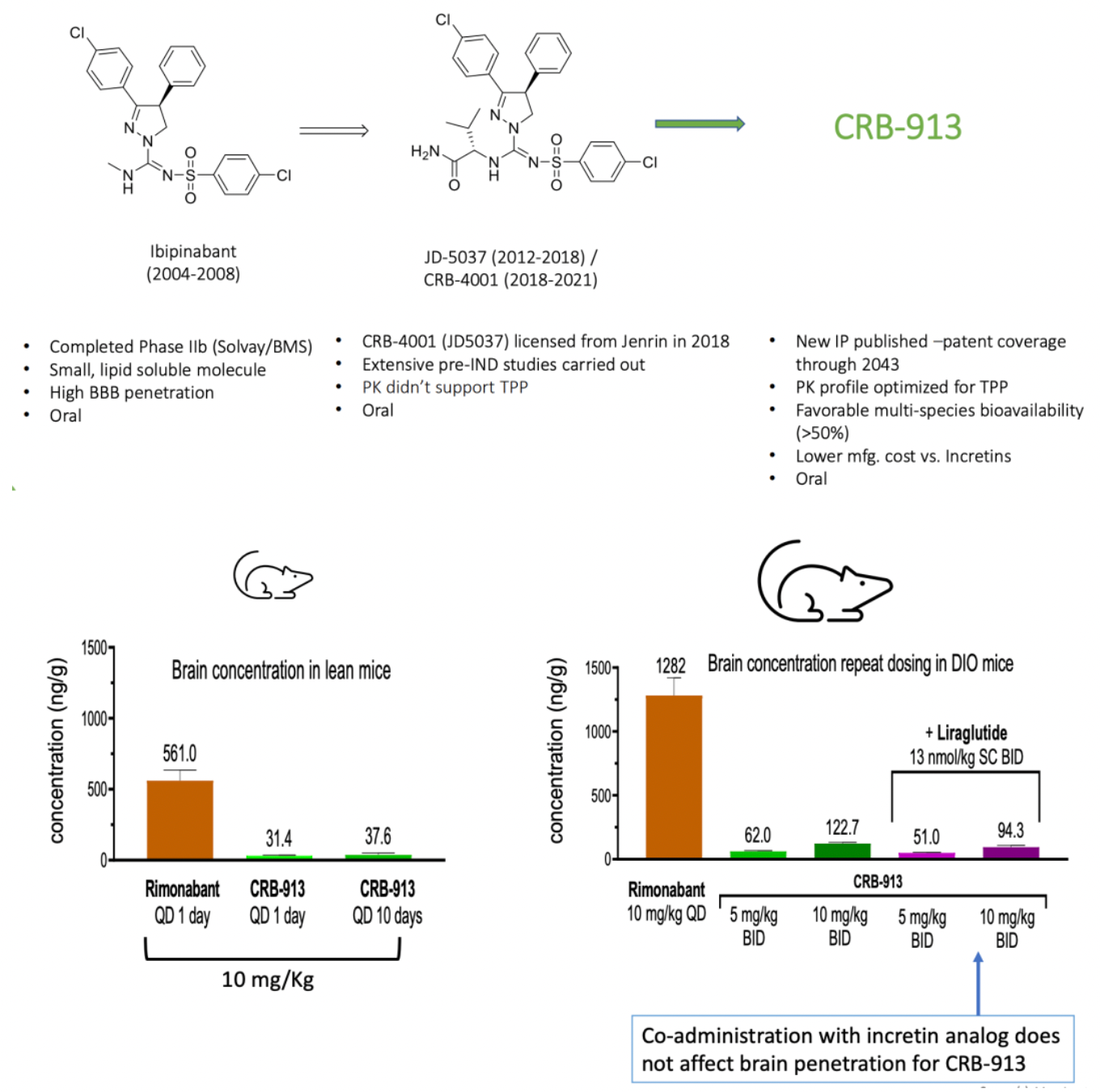
Ajulemic acid (Lenabasum) is another CB2 receptor agonist and IL-1β inhibitor developed by Corbus Pharmaceuticals in the United States, currently in Phase III clinical trials. Early clinical study results have indicated that Ajulemic acid can relieve pain in patients with chronic neuropathic pain without the clinically relevant psychoactive or physical side effects. Presently, this medication is being clinically investigated for a range of dermatological conditions, including discoid lupus erythematosus, dermatomyositis, systemic sclerosis, cystic fibrosis, and systemic lupus erythematosus.
CardiolRx (Cannabidiol) is a CB1/CB2 inverse agonist developed by Cardiol Therapeutics. Clinical indications under investigation for this compound include cardiovascular diseases, pericarditis, myocarditis, and heart failure.
CB1/CB2 Novel Drug Development Strategies
Despite the broad therapeutic potential of CB1/CB2, the development of candidate drugs is hindered by adverse reactions, rapid tolerance development, and the possibility of abuse. It has been proposed that ligands might differentiate the therapeutic effects from adverse reactions by producing biased signaling as a novel drug development strategy for the CB1/CB2 receptors. However, due to a lack of highly biased agonists, there is limited understanding of the biased signaling of the CB1 receptor.
Researchers are investigating the biased signaling characteristics of classical cannabinoid agonists and isomeric ligands in search of potential therapeutic advantages of CB1 receptor biased signaling in various pathological conditions.
CP55940 is a synthetic cannabinoid originally developed by Pfizer in 1974. It can mimic the effects of natural tetrahydrocannabinol, acting as an agonist for CB1/CB2 receptors. CP55940 is reported to be 45 times more potent than Δ9-THC and can be completely antagonized by Rimonabant. It is used as a research tool to study the endocannabinoid system but has never been studied clinically or sold on the market.
A study published in the "Journal of Molecular Pharmacology" (DOI: 10.1124/mol.115.101980) indicates that among the several downstream signaling pathways of the CB1 receptor, agonists THC and CP55940 show a preference for signaling through β-arrestin1, whereas the endogenous cannabinoids 2-AG and AEA exhibit a preference for signaling through Gαi/o. The researchers believe that enhancing Gαi/o-based endogenous cannabinoid signaling could have therapeutic effects for Huntington's disease. In contrast, drugs that preferentially target the β-arrestin1 signaling pathway could be detrimental to therapeutic signaling downstream of the CB1 receptor, especially in patients with Huntington's disease where CB1 receptor levels are already reduced.
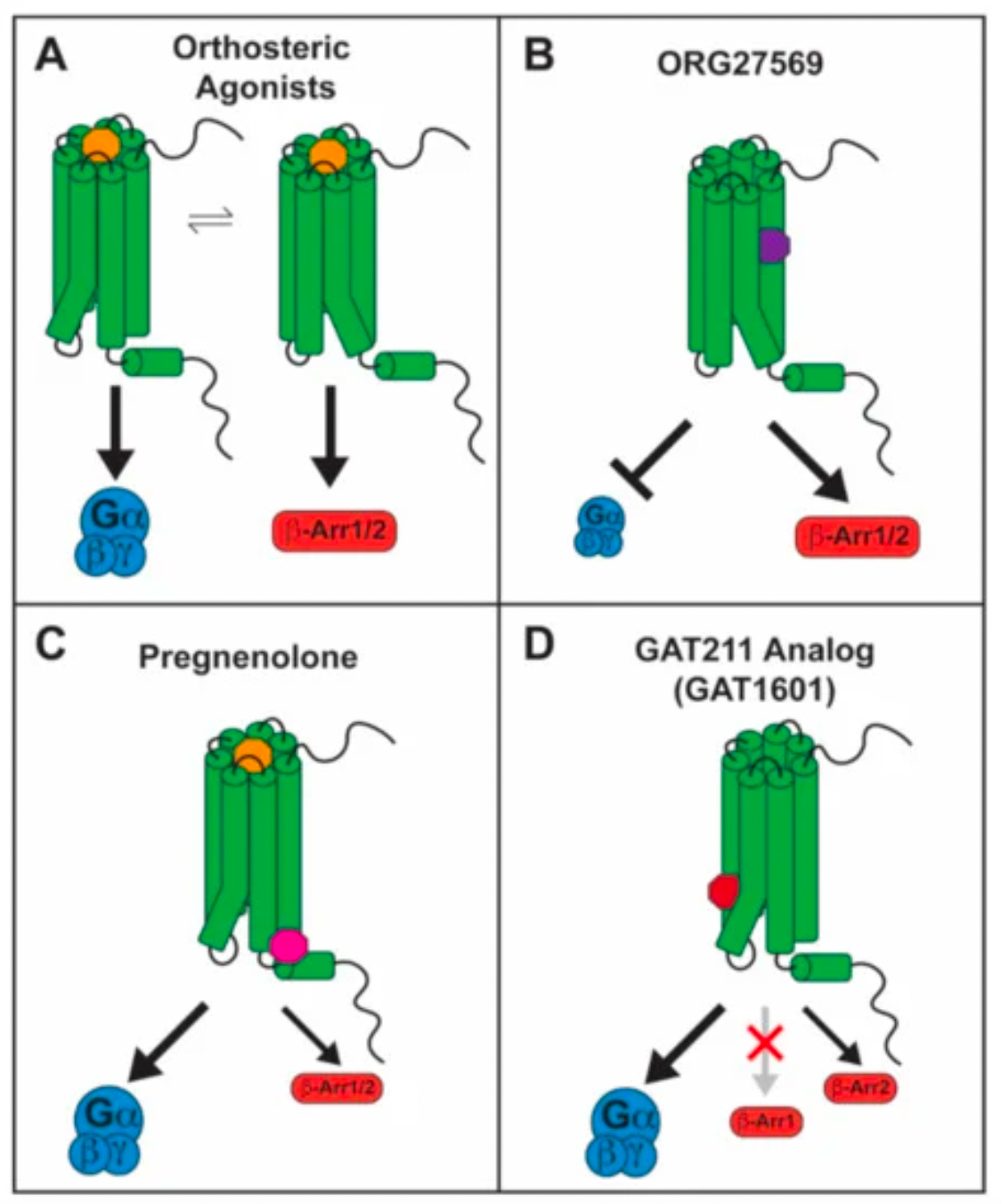
ORG27569 is an allosteric modulator targeting the CB1 receptor. It has been reported that ORG27569 is a ligand biased towards the β-arrestin1 signaling pathway of the CB1 receptor, acting as a negative allosteric modulator in terms of G protein signaling, and as a positive allosteric modulator in the context of β-arrestin1-mediated pERK1/2 signaling.
Recent research results published in the journal "Cell" (DOI: 10.1016/j.cell.2023.11.017) by Professor Zhang Yan and collaborators from Zhejiang University have filled a knowledge gap in structural biology regarding how the CB1 receptor couples to downstream β-arrestin signaling proteins.

By comparing the subtle structural differences between the inactive state bound to an antagonist, the active state with agonist-receptor-Gi protein binding, and the state with agonist-receptor-β-arrestin1 binding, the researchers seem to have found some clues about the bias of downstream CB1 receptor activation. This may provide a structural basis for designing safer analgesic and anti-anxiety medications.